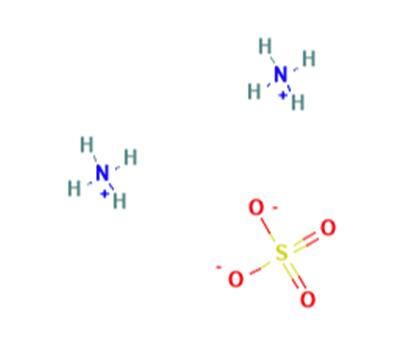
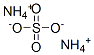Uses of Ammonium sulfate
Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia,it is widely used as a fertilizer for alkaline soils.

Properties
Ammonium sulfate becomes ferroelectric at temperatures below –49.5 °C. At room temperature it crystallises in the orthorhombic system, with cell sizes of a = 7.729 Å, b = 10.560 Å, c = 5.951 Å.
Uses
The primary use of ammonium sulfate is as a fertilizer for alkaline soils. In the soil the ammonium ion is released and forms a small amount of acid, lowering the pH balance of the soil, while contributing essential nitrogen for plant growth. The main disadvantage to the use of ammonium sulfate is its low nitrogen content relative to ammonium nitrate, which elevates transportation costs.
It is also used as an agricultural spray adjuvant for water-soluble insecticides, herbicides, and fungicides. There, it functions to bind iron and calcium cations that are present in both well water and plant cells. It is particularly effective as an adjuvant for 2,4-D (amine), glyphosate, and glufosinate herbicides.
Preparation
Ammonium sulfate is made by treating ammonia with sulfuric acid:
2 NH3 + H2SO4 → (NH4)2SO4
A mixture of ammonia gas and water vapor is introduced into a reactor that contains a saturated solution of ammonium sulfate and about 2 to 4% of free sulfuric acid at 60 °C. Concentrated sulfuric acid is added to keep the solution acidic, and to retain its level of free acid. The heat of reaction keeps reactor temperature at 60 °C. Dry, powdered ammonium sulfate may be formed by spraying sulfuric acid into a reaction chamber filled with ammonia gas. The heat of reaction evaporates all water present in the system, forming a powdery salt. Approximately 6,000 million tons were produced in 1981.
Ammonium sulfate also is manufactured from gypsum (CaSO4·2H2O). Finely divided gypsum is added to an ammonium carbonate solution. Calcium carbonate precipitates as a solid, leaving ammonium sulfate in the solution.
(NH4)2CO3 + CaSO4 → (NH4)2SO4 + CaCO3
Ammonium sulfate occurs naturally as the rare mineral mascagnite in volcanic fumaroles and due to coal fires on some dumps.
You may like
Related articles And Qustion
Lastest Price from Ammonium sulfate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7783-20-2
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $100.00-75.00/kg2025-04-21
- CAS:
- 7783-20-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000