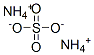Synthesis of Ammonium sulfate
General description
Ammonium sulfate is an inorganic sulfate salt obtained by reaction of sulfuric acid with two equivalents of ammonia. A high-melting (decomposes above 280℃) white solid which is very soluble in water (70.6 g/100 g water at 0℃; 103.8 g/100 g water at 100℃), it is widely used as a fertilizer for alkaline soils. It has a role as a fertilizer. It is an ammonium salt and an inorganic sulfate salt. Due to bound lipid and/or detergents, ammonium sulfate precipitates have lower density than protein-only precipitates. Duringcentrifugation, these precipitates will often float to the top of tube rather than pelleting;the use of swing-out rotors is recommended. Crystallization is a traditional method of protein purification. Jakoby (1971) describes a general method that involves extracting (NH4)2SO4-precipitated protein with successively dilute (NH4)2SO4 solutions at low temperature. As mentioned above, (NH4)2SO4 and other neutral salts stabilize proteins by preferential solvation. Proteins are often stored in (NH4)2SO4, which inhibits bacterial growth and contaminating protease activities.
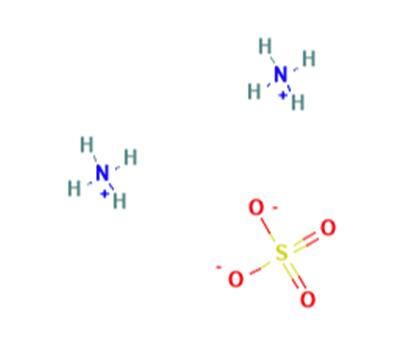
Figure 1 the structure of Ammonium sulfate
Application
Ammonium sulfate is an excellent nitrogen fertilizer, suitable for general soil and crops. It can make branches and leaves grow vigorously, improve fruit quality and yield, and enhance crop disaster resistance. It can be used as base fertilizer, top dressing and seed fertilizer. It reacts with salt to generate ammonium chloride, reacts with aluminum sulfate to generate ammonium alum, and generates refractory materials together with boric acid. Adding it to the plating solution increases conductivity. It is also a catalyst for food sauce color, a nitrogen source for cultivating yeast in the production of fresh yeast, a dyeing assistant for acid dyes, and a deliming agent for leather. In addition, it can also be used in beer brewing, chemical reagents and battery production. Another important role is the mining of rare earths. Mining is carried out with ammonium sulfate as the raw material, and the rare earth elements in the ore are exchanged through ion exchange, and then the leaching solution is collected to remove impurities, precipitate, press, and burn to form rare earth ore. 1 ton of rare earth ore requires about 5 tons of ammonium sulfate. There are also many biological uses, mostly used in the protein purification process, because ammonium sulfate is an inert substance and is not easy to react with other biologically active substances, which can protect the protein activity to the greatest extent during the purification process. In addition, ammonium sulfate is extremely soluble. A high-salt environment can be created in preparation for protein precipitation and subsequent high-salt purification[1].
Mechanism of action
After neutralization with ammonium hydroxide and sulfuric acid, it is obtained by crystallization, centrifugation and drying. Neutralization method Ammonia and sulfuric acid are neutralized at about 100°C, and the resulting ammonium sulfate crystal slurry is centrifuged and dried to obtain finished ammonium sulfate. Its 2NH3+H2SO4→(NH4)2SO4 recovery method is obtained by recovering ammonia gas from coke oven gas, and then neutralizing it with sulfuric acid.
Synthesis
In industry, it is obtained by direct neutralization reaction of ammonia and sulfuric acid. It is not used much. It is mainly used to absorb by-products or exhaust gas in industrial production with sulfuric acid or ammonia water (such as sulfuric acid to absorb ammonia in coke oven gas, and ammonia water to absorb smelter). Sulfur dioxide in flue gas, ammonia in Kaplan production or sulfuric acid waste liquid in titanium dioxide production by sulfuric acid method). There are also ammonium sulfate produced by gypsum method (with natural gypsum or phosphogypsum, ammonia, carbon dioxide as raw materials[2].
References
1.Fishman J. B. & Berg E. A., "Ammonium Sulfate Fractionation of Antibodies," Cold Spring Harbor Protocols, Vol.2018, No.6(2018), p.t99119.
2.Duong-Ly K. C. & Gabelli S. B., "Salting out of proteins using ammonium sulfate precipitation," Methods Enzymol, Vol.541(2014), pp.85-94.
You may like
Related articles And Qustion
See also
Lastest Price from Ammonium sulfate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7783-20-2
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $100.00-75.00/kg2025-04-21
- CAS:
- 7783-20-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000