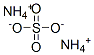What is Ammonium Sulfate?
Identification
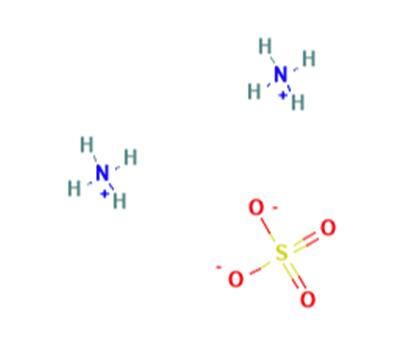
Product Name: Ammonium sulfate
Synonyms: (NH4)2 SO4;actamaster;ammoniumsulfate(2:1);Wasfcaguite ;AMMONIUM SULPHATE SOLUTION NO 2;AMMONIUM SULPHATE TECHNOLOGY;AMONIUM SHLPHATE, TECH;AMMNIUM SULPHATE
CAS: 7783-20-2
MF: H8N2O4S
MW: 132.14
EINECS: 231-984-1
Properties
Melting point >280 °C (dec.) (lit.)
Density 1.77 g/mL at 25 °C (lit.)
Vapor pressure <1 Pa (25 °C)
Refractive index n20/D 1.396
Fp 26 °C
Storage temp. room temp
Solubility H2O: 1 M at 20 °C, clear, colorless
Form Solid
Specific Gravity 1.769
Color Yellow to orange
Odor Slight odor of ammonia
PH 5.0-6.0 (25℃, 1M in H2O)
PH Range 5 - 6
Water Solubility 77 g/100 mL (25 ºC)
Ammonium sulfate [(NH₄)₂ SO₄] was one of the first and most widely used nitrogen (N) fertilizers for crop production. It’s now less common but especially valuable where both N and sulfur (S) are required. Its high solubility provides versatility for a number of agricultural applications.
Ammonium sulfate (sometimes abbreviated as AS or AMS) has been produced for more than 150 years. Initially, it was made from ammonia released during manufacturing coal gas (used to illuminate cities) or from coal coke used to produce steel. Today, manufacturers make it by reacting sulfuric acid with heated ammonia. To get the crystal size best suited for the application, they control the reaction conditions by screening and drying the particles until achieving the desired size. Some materials are coated with a conditioner to reduce dust and caking.
Use in Fertilizers
Ammonium sulfate is used most commonly as an artificial fertilizer for alkaline soils. When introduced into damp soil, an ammonium ion is released. This creates a small amount of acid, which lowers the pH balance of the soil. It also contributes nitrogen, which aids in plant growth. It dissolves relatively slowly, which makes it cheaper than some other artificial fertilizers. Ammonium sulfate is also used as an herbicide because it will burn the leaves of plants and either kill them outright or at least weaken them for easy removal.
Other Uses
This compound is used in the production of printed circuit boards. It’s also in flame retardant materials because it lowers the combustion temperature and increases the production of residues or chars. Ammonium sulfate activates yeast, so it helps to get industrially produced bread to rise, and it’s also a general-purpose food additive. Finally, it plays an important role in developing vaccines during the purification process. The DTap vaccine, which protects children from diphtheria, tetanus, and whooping cough, uses ammonium sulfate for this purpose.
Hazards of Use
Ammonium sulfate is potentially dangerous to both people and the environment, so it requires care in its use. It can cause severe irritation and inflammation of the respiratory tract if inhaled. Eating or drinking ammonium sulfate will cause irritation in the gastrointestinal tract like nausea, vomiting, and diarrhea, although it isn’t toxic unless consumed in large quantities. Contact with the skin or eyes will cause irritation, redness, itching, and pain. It may also be a neurotoxin, meaning it can cause confusion and behavioral changes.
You may like
Related articles And Qustion
Lastest Price from Ammonium sulfate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 7783-20-2
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $100.00-75.00/kg2025-04-21
- CAS:
- 7783-20-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000