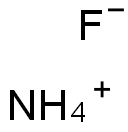Ammonium fluoride:Lewis structure,Preparation,Uses and Hazards
Ammonium fluoride (NH4F) is a white, hydroscopic, crystal. The solutions are clear, colorless liquids that have a slightly sharp, pungent odor.
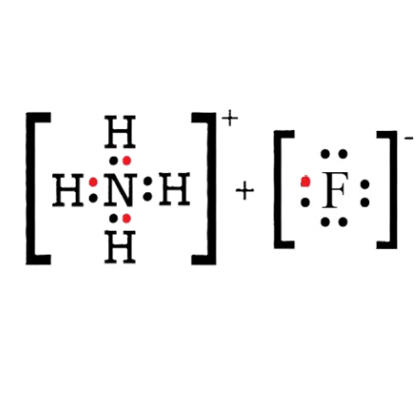
Lewis structure
Preparation
Ammonium fluoride is made by mixing ammonia and anhydrous hydrogen fluoride (liquid) and then drying to form flakes.
HF + NH3 → NH4 F
Uses
Ammonium fluoride (AF) is used primarily for oil well acidification and metal processing. It is also used in the production of electronic components.
Hazards
Ammonium fluoride is a corrosive chemical and contact can severely irritate and burn the skin and eyes causing possible permanent eye damage. Breathing ammonium fluoride can severely irritate and burn the nose, throat, and lungs, causing nosebleeds, cough, wheezing and shortness of breath. Contact of ammonium fluoride with water or moist skin can release hydrofluoric acid, a very dangerous acid. Ammonium fluoride crystals are hydroscopic (absorb moisture from the air). They also sublime (vaporize without going into a liquid state).
Environmental Information
Ammonium fluoride is not known to bioaccumulate or persist in the environment more than a few days. However, it will decompose in moist environments liberating hydrofluoric acid and ammonia.
You may like
Lastest Price from Ammonium fluoride manufacturers

US $0.00-0.00/KG2025-12-10
- CAS:
- 12125-01-8
- Min. Order:
- 500KG
- Purity:
- 98%
- Supply Ability:
- 500tons

US $10.00/kg2025-04-21
- CAS:
- 12125-01-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt