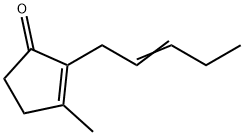
Jasmone: A Multifaceted Molecule in Plant - Insect Interactions and Beyond
Jasmone, is well known as a component of plant volatiles, and its release can be induced by damage, for example during insect herbivory. Using the olfactory system of the lettuce aphid to investigate volatiles from plants avoided by this insect, jasmone was found to be electrophysiologically active and also to be repellent in laboratory choice tests.

Toxicologic and dermatologic review of cis-jasmone
A toxicologic and dermatologic review of cis-jasmone when used as a fragrance ingredient is presented. cis-Jasmone is a member of the fragrance structural group ketones cyclopentanones and cyclopentenones. The common characteristic structural element of the group members is a cyclopentanone or cyclopentenone ring with a straight or branched chain alkane or alkene substituent. This review contains a detailed summary of all available toxicology and dermatology papers that are related to this individual fragrance ingredient and is not intended as a stand-alone document. Available data for cis-jasmone were evaluated then summarized and includes physical properties, acute toxicity, skin irritation, mucous membrane (eye) irritation, skin sensitization, phototoxicity, photoallergy, and genotoxicity data.[1]
cis-jasmone is a fragrance ingredient used in many fragrance mixtures. It may be found in fragrances used in decorative cosmetics, fine fragrances, shampoos, toilet soaps and other toiletries as well as in non-cosmetic products such as household cleaners and detergents. It is a pale yellowish or pale straw-colored oily liquid with a diffusive, warm-spicy, somewhat fruity, but in dilution more floral odor of good tenacity. The worldwide volume of use for cis-jasmone is in the region of 1–10 metric tons per year. The LD50 when cis-jasmone was administered orally to rats was approximately 5 g/kg. cis-Jasmone was administered by gavage to 10 rats at a dose of 5 g/kg followed by a 14 day observation period. Mortality was seen in 5 animals by day 2. All rats were in a coma by 3 h after dosing. Gross necropsy showed darkened livers, yellow material in the stomach and red intestines in four animals and red lungs in one animal. The LD50 when cis-jasmone was administered orally to rats was reported to be 4.3 g/kg (3.58 – 5.16 g/kg). cis-Jasmone was administered by gavage to 9 rats at doses of 3.5, 4.0 and 4.5 g/kg. Mortality was seen 3, 3, and 6 of 9 rats with increasing dose.
In an open epicutaneous test in guinea pigs, 0.1 ml of 8% cis-jasmone was applied daily for 3 weeks with the site uncovered. Following induction, the animals were challenged on days 21 and 35 with 0.025 ml of 8% cis-jasmone. The sites were scored 24, 48 and 72 h after exposure. A challenge concentration of 8% cis-jasmone was not sensitizing to guinea pigs. In an open epicutaneous test in guinea pigs, 0.1 ml cis-jasmone was applied daily for 3 weeks with the site uncovered at concentrations of 100%, 30%, 10% and 3% in acetone; 4 animals were used per group. Following induction, the animals were challenged on days 21 and 35 with 0.025 ml of cis-jasmone. The sites were scored 24, 48 and 72 h after exposure. None of the concentrations test induced sensitization in any of the animals tested.
An odorant receptor mediates the attractiveness of cis-jasmone
Parasitic wasps rely on olfaction to locate their hosts in complex chemical environments. Odorant receptors (ORs) function together with well-conserved odorant coreceptors (ORcos) to determine the sensitivity and specificity of olfactory reception. Campoletis chlorideae (Hymenoptera: Ichneunmonidae) is a solitary larval endoparasitoid of the cotton bollworm, Helicoverpa armigera, and some other noctuid species. To understand the molecular basis of C. chlorideae's olfactory reception, we sequenced the transcriptome of adult male and female heads (including antennae) and identified 211 OR transcripts, with 95 being putatively full length. The tissue expression profiles, as assessed by reverse-transcription PCR, showed that seven ORs were expressed only or more highly in female antennae. Their functions were analysed using the Xenopu slaevis oocyte expression system and two-electrode voltage-clamp recordings. CchlOR62 was tuned to cis-jasmone, which was attractive to female C. chlorideae adults and H. armigera larvae in the subsequent behavioural assays. Further bioassays using caged plants showed that the parasitism rate of H. armigera larvae by C. chlorideae on cis-jasmone-treated tobacco plants was higher than on the control plants. Thus, cis-jasmone appears to be an important infochemical involved in the interactions of plants, H. armigera and C. chlorideae, and CchlOR62 mediates the attractiveness of cis-jasmone to C. chlorideae.[2]
cis-jasmone as an insect semiochemical and in plant defense
cis-Jasmone, or (Z)-jasmone, is well known as a component of plant volatiles, and its release can be induced by damage, for example during insect herbivory. Using the olfactory system of the lettuce aphid to investigate volatiles from plants avoided by this insect, (Z)-jasmone was found to be electrophysiologically active and also to be repellent in laboratory choice tests. In field studies, repellency from traps was demonstrated for the damson-hop aphid, and with cereal aphids numbers were reduced in plots of winter wheat treated with (Z)-jasmone. In contrast, attractant activity was found in laboratory and wind tunnel tests for insects acting antagonistically to aphids, namely the seven-spot ladybird and an aphid parasitoid. When applied in the vapor phase to intact bean plants, (Z)-jasmone induced the production of volatile compounds, including the monoterpene (E)-β-ocimene, which affect plant defense, for example by stimulating the activity of parasitic insects. These plants were more attractive to the aphid parasitoid in the wind tunnel when tested 48 h after exposure to (Z)-jasmone had ceased. This possible signaling role of (Z)-jasmone is qualitatively different from that of the biosynthetically related methyl jasmonate and gives a long-lasting effect after removal of the stimulus. Differential display was used to compare mRNA populations in bean leaves exposed to the vapor of (Z)-jasmone and methyl jasmonate. One differentially displayed fragment was cloned and shown by Northern blotting to be up-regulated in leaf tissue by (Z)-jasmone. This sequence was identified by homology as being derived from a gene encoding an α-tubulin isoform.[3]
The roles described in this study have not previously been demonstrated for (Z)-jasmone, although its release from plants can be induced by damage, including that caused during feeding by lepidopterous larvae. (Z)-Jasmone is a very volatile product of further catabolism of jasmonic acid; in fact, in elegant work on the metabolism of jasmonic acid, the question was raised as to whether (Z)-jasmone is a biologically inactive sink for the jasmonic acid pathway induced during plant defense. As a noninduced flower volatile, (Z)-jasmone is well known as an attractant of pollinating insects, although it also has been shown to attract the phytophagous Japanese beetle, Popillia japonica (Scarabaeidae), in field trapping trials.
Scientists propose that rather than (Z)-jasmone being considered as another lipoxygenase-derived volatile and a possible sink for jasmonic acid, it should be viewed as a potential airborne plant signal relating to other aspects of plant signaling, although we have not yet demonstrated a role as a signal acting between plants. It also should be noted that (Z)-jasmone is more volatile than methyl jasmonate and, as such, could be a more effective signal compound. We have demonstrated that, far from being biologically inactive, (Z)-jasmone has activity at all three trophic levels investigated in this study. The work reported here identifies a compound significantly inducing production of these types of compounds as an airborne signal. (Z)-Jasmone, an extremely benign component of perfumes and floral volatiles, eventually may find use in inducing gene expression for proteins with functions beyond the secondary metabolism involved in insect/plant interactions, because (Z)-jasmone responsive promoters could be useful tools in the molecular genetic engineering of novel traits in plants.
References
[1]Scognamiglio J, Jones L, Letizia CS, Api AM. Fragrance material review on cis-jasmone. Food Chem Toxicol. 2012 Oct;50 Suppl 3:S613-8.
[2]Sun YL, Dong JF, Ning C, Ding PP, Huang LQ, Sun JG, Wang CZ. An odorant receptor mediates the attractiveness of cis-jasmone to Campoletis chlorideae, the endoparasitoid of Helicoverpa armigera. Insect Mol Biol. 2019 Feb;28(1):23-34.
[3]Birkett MA, Campbell CA, Chamberlain K, Guerrieri E, Hick AJ, Martin JL, Matthes M, Napier JA, Pettersson J, Pickett JA, Poppy GM, Pow EM, Pye BJ, Smart LE, Wadhams GH, Wadhams LJ, Woodcock CM. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):9329-34.
You may like
Lastest Price from Jasmone manufacturers

US $6.00/kg2025-04-21
- CAS:
- 488-10-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000

US $0.00-0.00/KG2025-04-21
- CAS:
- 488-10-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt