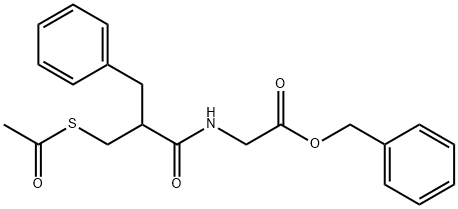
Racecadotril: A Promising Solution for Acute Infectious Diarrhea Management
Racecadotril is a prodrug that, after oral administration, is converted into thiorphan by hydrolysis and it exerts its effects acting on enkephalinase, a peptidase located in the cell membrane, in different tissues, but mainly in the small intestine mucosa. This enzyme is involved in the metabolism of enkephalins, endogenous opioids that in physiologic conditions contribute to maintaining the secretion/absorption balance in the small intestine.

Racecadotril in the management of diarrhea
Acute infectious diarrhea (AID) represents an important clinical entity both regarding morbidity and mortality rates, even in industrialized countries, and it leads to one of the major public health burdens, among gastroenterological diseases, with significant healthcare costs. Oral rehydration solution is the cornerstone of the therapy, but despite its proven efficacy in avoiding dehydration, it is still underused as it does not reduce the duration of diarrhea; hence, it is perceived as ineffective by caregivers. In this narrative review, we collected literature regarding the use of racecadotril, deeply discussing its role in the treatment of AID in both adults and children. Racecadotril has been studied in wide populations of patients, in many countries, and in different clinical settings. Its effectiveness in reducing the stool output and the duration of diarrhea has been proven, not only in the early phase of the disease. Racecadotril has been shown to increase the likelihood of home management of AID, to reduce hospitalizations and parenteral rehydration needs resulting in healthcare costs reduction. The current new formulations require only two-daily doses for adults and the pediatric syrup should simplify its use.[1]
Acute diarrhea is one of more frequent infectious diseases, with risk of dehydration if not adequately treated especially in children and the elderly, and this impacts on the healthcare costs beyond on the caregivers. Racecadotril, the first and only intestinal antisecretory drug, acting on the abnormal intestinal hypersecretion, decreases the loss of water and electrolytes from the gut, so reducing the dehydration risk, it increases the likelihood of home management of diarrhea. The efficacy of racecadotril has been demonstrated in all settings (inpatients, outpatients, and community-based), in patients of all ages (children, adults, and the elderly), in many countries both of high-income and low-middle-income. In addition, the safety of racecadotril has always been comparable to placebo and better than loperamide. Racecadotril was approved in France in 1992, and it is now available in many countries in Europe, Latin America, Asia and Middle East, and Africa, even as an over-the-counter product, for the treatment of AID. The pivotal studies on the efficacy of racecadotril date back to the early 2000s and some of them measured the stool output, defined by the WHO as the most significant and reliable parameter to evaluate the effectiveness of anti-diarrheal drugs. Since then, many studies confirmed racecadotril efficacy, both in adults and in children, in all clinical settings. Despite racecadotril being a drug with a good safety and efficacy profile (see the sections below), it is an underestimated therapeutic option.
Racecadotril, an enkephalinases inhibitor seems to be an effective and safe option in AID associated with a very low number of DDI. Its efficacy is superior to probiotics and, in children, it improves rehydration efficacy and increases the probability to manage successfully at home the episodes of AID. Racecadotril effectiveness has been confirmed in both children and adults with AID, in inpatient and outpatient settings, in several clinical trials, meta-analyses, and systematic reviews done all over the world, from high-income to middle- and low-income countries. Furthermore, a favorable racecadotril cost/effectiveness balance should encourage its use in clinical practice. The actual availability of an adult formulation requiring only two-daily doses and of a pediatric syrup should simplify its use.
Pharmacodynamics, Pharmacokinetics, and Clinical Effects of Racecadotril
Racecadotril is a low potency inhibitor of NEP, but upon oral administration it is rapidly and effectively metabolized to the potent NEP inhibitor thiorphan, with the latter not exhibiting penetration into the central nervous system. NEP inhibition affects the abundance of several endogenous peptides with enkephalins and ANP apparently being most important. Elevated exposure to ANP appears to underly most cardiovascular effects of racecadotril; while these tend to be beneficial they appear quantitatively insufficient to warrant therapeutic use in comparison to other available drug classes. Elevation of enkephalin exposure appears to underly most central nervous effects, most notably analgesia, but the pain relieving effects are inconsistent across animal models. Increased exposure to peripheral endogenous enkephalins appears to underly the gastro-intestinal racecadotril effects. Most prominent among them is an antisecretory effect in the gut which, in contrast to direct μ-opioid receptor agonists, occurs in the absence of effects on gastro-intestinal transit time. [2]
The clinical correlate of these findings is therapeutic efficacy against acute diarrhea in adults and children with a tolerability profile similar to that of placebo. In multiple direct comparative studies in different patients populations (children, adults, elderly), countries (Western Europea, Latin Amercia, Asia), and settings (out-patients, in-patients, nursing home residents) racecadotril was at least as effective as loperamide, and in several of those studies exhibited significantly better tolerability than loperamide. Most notably, rebound constipation was consistently less frequent with racecadotril than with loperamide; while this is primarily a tolerability benefit, it may also be relevant with regard to the efficacy of clearance of infectious organisms as demonstrated in one study. Of note, study designs and particularly treatment endpoints differed considerably between studies. This can be seen as a weakness because it makes inter-study comparisons more difficult; however, it can also be seen as a benefit because consistent therapeutic effects across so many different settings witness rather robust efficacy and tolerability. While additional studies appear warranted several guidelines, specifically in pediatric indications, now recommend including racecadotril in the management of acute diarrhea. Whether other forms of diarrhea, e.g., in the context of cancer chemotherapy, also benefit from racecadotril treatment is not fully clear.
Racecadotril : A Novel Antidiarrheal
Oral rehydration has been the mainstay of treatment of diarrhea, however it does not reduce the frequency of stools or the number of diarrheal days. Antimotility drugs like loperamide have a limited role because of side effects. Potentiation of the effects of endogenous enkephalin activity by enkephalinase inhibition has produced a safe and effective anti secretory drug, Racecadotril. Racecadotril is rapidly converted in the body to thiorphan, a potent enkephalinase inhibitor. Enkephalins are endogenous opioid peptides secreted by myenteric and sub mucosal neurons in the digestive tract. Racecadotril is administered by the oral route, is well absorbed from the intestinal tract and is rapidly converted to its active metabolite thiorphan. Peak plasma levels are attained in about an hour and half life of the drug is three hours. Data on safety in pregnancy, lactation and renal/hepatic insufficiency is inadequate, which requires care in the usage.[3]
The efficacy of racecadotril in acute diarrhoea is not associated with adverse gastrointestinal effects and fewer patients on racecadotril therapy suffered from abdominal distension following treatment (5.6 vs 18.2% on placebo). It caused significantly less constipation after resolution of diarrhea than loperamide. Incidence of itching was higher in racecadotril than loperamide group. Racecadotril does not enter the central nervous system (CNS) thus it lacks any potential for neurotoxicity; however in children below two years of age where blood brain barrier is immature it can cause depression. Caution is also advocated in using racecadotril in disorders of carbohydrate intolerance due to the presence of saccharose as an excipient.
References
[1]Manfredi M, Marcianò G, Iuliano S, Leo F, Gallelli L. Racecadotril in the management of diarrhea: an underestimated therapeutic option? Therap Adv Gastroenterol. 2025 Jan 6;18:17562848241310423.
[2]Eberlin M, Mück T, Michel MC. A comprehensive review of the pharmacodynamics, pharmacokinetics, and clinical effects of the neutral endopeptidase inhibitor racecadotril. Front Pharmacol. 2012 May 30;3:93.
[3]Singh N, Narayan S. Racecadotril : A Novel Antidiarrheal. Med J Armed Forces India. 2008 Oct;64(4):361-2.
You may like
Lastest Price from Racecadotril manufacturers

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 81110-73-8
- Min. Order:
- 1KG
- Purity:
- 98%-102%
- Supply Ability:
- 1000KG

US $0.00-0.00/kg2025-04-21
- CAS:
- 81110-73-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500kg