Hydrocortisone Acetate: Chemical Properties, Applications, and Storage in Modern Pharmaceuticals
Introduction to Hydrocortisone Acetate
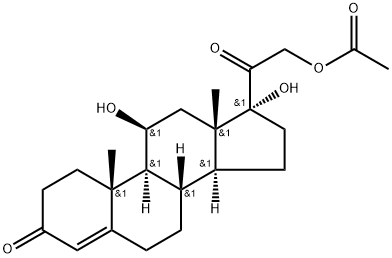
Hydrocortisone acetate is the synthetic acetate ester form of hydrocortisone, which is a corticosteroid with anti-inflammatory and immunosuppressive properties. Hydrocortisone acetate initially binds to cytoplasmic glucocorticoid receptors; Then the receptor-ligand complex translocates to the nucleus, where it initiates transcription of genes encoding anti-inflammatory mediators such as cytokines and lipoproteins. Lipodermin inhibits phospholipase A2, thereby blocking the release of arachidonic acid from membrane phospholipids and inhibiting the synthesis of prostaglandins and leukotrienes. Let's get to know Hydrocortisone acetate correctly together.

Figure 1 Characteristics of Hydrocortisone acetate
Application of Hydrocortisone Acetate
Hydrocortisone acetate is a synthetic hormone substance widely used in the medical field. This article will introduce the medical applications of hydrocortisone acetate.
1. Treatment of skin diseases
Hydrocortisone acetate can be used to treat various skin diseases, such as eczema, psoriasis, and urticaria. As a hormone medication, it can alleviate symptoms such as skin inflammation, redness, and itching, providing relief for patients.
2. Treatment of allergic rhinitis
By inhibiting the release of inflammatory factors, hydrocortisone acetate can reduce the inflammatory response in the nasal cavity and is therefore used to treat allergic rhinitis. Allergic rhinitis often causes discomfort symptoms such as sneezing and runny nose, and the use of hydrocortisone acetate can effectively alleviate these symptoms.
3. Treatment of arthritis
Hydrocortisone acetate can be used to treat rheumatoid arthritis, osteoarthritis, and other diseases. It can alleviate symptoms such as joint swelling and pain, while also slowing down the progression of arthritis.
4. Treatment of asthma
By inhibiting the activity of inflammatory cells, hydrocortisone acetate can alleviate symptoms such as difficulty breathing during asthma attacks. Asthma is a common respiratory disease, and the use of hydrocortisone acetate provides a more effective treatment option for asthma patients.
5. Clinical diagnosis
Hydrocortisone acetate can be used as an endocrine function test drug to examine the function of the hypothalamic-pituitary adrenal axis. In clinical practice, the functional status of this axis is usually evaluated by measuring the level of cortisol in the blood.
Hydrocortisone acetate has a wide range of applications in medicine. It can be used to treat skin diseases, allergic rhinitis, arthritis, and asthma, and can also be used as an endocrine function test drug.
Precautions for Hydrocortisone Acetate
Hydrocortisone Acetate has a relatively low toxicity when used as prescribed. However, overuse or misuse can lead to side effects such as skin thinning, delayed wound healing, and possible systemic effects if absorbed into the bloodstream in large amounts. Long-term use of corticosteroids, including hydrocortisone acetate, has been associated with increased risks of osteoporosis, high blood pressure, and diabetes. The compound may irritate the application site, particularly when used in high concentrations. There is no strong evidence to suggest that hydrocortisone acetate is carcinogenic, but prolonged or excessive use should be monitored by a healthcare professional. It should be stored in a cool, dry place, away from heat and direct sunlight, to preserve its stability. The ideal temperature range for storage is between 15°C and 30°C. It should be kept in tightly sealed containers, and care should be taken to avoid contamination. Hydrocortisone acetate should be kept out of reach of children and only used under medical supervision.
For safe use, hydrocortisone acetate must be applied according to prescribed instructions, and patients should follow their doctor's recommendations regarding dosage and frequency. It should not be applied to large areas of the body or used for extended periods without medical supervision, as this can lead to systemic absorption and potential side effects. Always store the product in a secure, well-ventilated environment, away from sources of contamination or incompatible substances. Disposal of unused or expired products should follow local regulations to ensure environmental safety.
![Article illustration]() Reference
Reference
[1] Raghavan, S. L., et al. "Crystallization of hydrocortisone acetate: influence of polymers."International journal of pharmaceutics212.2 (2001): 213-221.
[2] Fini, Adamo, et al. "Control of transdermal permeation of hydrocortisone acetate from hydrophilic and lipophilic formulations."AAPS PharmSciTech9 (2008): 762-768.
References:
[1] S.L RAGHAVAN . Crystallization of hydrocortisone acetate: influence of polymers[J]. International Journal of Pharmaceutics, 2001, 212 2: 153-304. DOI:10.1016/S0378-5173(00)00610-4.[2] ADAMO FINI. Control of Transdermal Permeation of Hydrocortisone Acetate from Hydrophilic and Lipophilic Formulations[J]. AAPS PharmSciTech, 2008, 9 3. DOI:10.1208/s12249-008-9107-z.
Lastest Price from Hydrocortisone acetate manufacturers

US $55.00-40.00/kg2025-06-13
- CAS:
- 50-03-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 2000kgs

US $5.00-0.50/KG2025-05-30
- CAS:
- 50-03-3
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS