Application research of 1,1,2,2-Tetrafluoroethyl 2,2,2-trifluoroethyl ether
Introduction
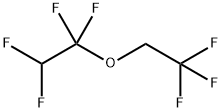
1,1,2,2-Tetrafluoroethyl 2,2,2-trifluoroethyl ether (Figure 1), also known as perfluoroethyl trifluoromethyl ether, is a fluorinated compound with unique chemical properties and extensive applications. Containing fluorine in its chemical structure, 1,1,2,2-tetrafluoroethyl 2,2,2-trifluoroethyl ether exhibits exceptional chemical inertness and thermal stability, enabling it to exist stably under extreme conditions such as high temperature, high pressure, and strong acid-base environments while being resistant to chemical reactions. Due to the extremely high electronegativity of the fluorine atoms in its molecule, 1,1,2,2-tetrafluoroethyl 2,2,2-trifluoroethyl ether possesses excellent dielectric properties and insulation performance, making it an ideal insulating material widely used in electronic devices, power equipment, aerospace, and other fields. 1,1,2,2-Tetrafluoroethyl 2,2,2-trifluoroethyl ether also features low surface tension and viscosity, endowing it with superior wettability and fluidity. Consequently, it is extensively employed as a lubricant and wetting agent. In certain specialized processes, 1,1,2,2-tetrafluoroethyl 2,2,2-trifluoroethyl ether can effectively reduce friction between objects and provide excellent lubrication. Additionally, 1,1,2,2-tetrafluoroethyl 2,2,2-trifluoroethyl ether demonstrates good corrosion resistance and chemical resistance, rendering it suitable for applications requiring resistance to acid-base corrosion. Beyond the aforementioned fields, 1,1,2,2-tetrafluoroethyl 2,2,2-trifluoroethyl ether is also widely utilized in material surface treatment, textile printing and dyeing, inks, coatings, and other industries.

Locally concentrated surface-active ionic liquids preparation
Diluting the surface-active ionic liquid 1-butyl-3-methylimidazolium 1,4-bis-2-ethylhexylsulfosuccinate (BMIM AOT) with the non-solvating diluent 1,1,2,2-tetrafluoroethyl-2,2,2-trifluoroethyl ether (TFTFE) will reduce viscosity while preserving essential nanostructures and electrochemical properties, creating locally concentrated ionic liquids (LCILs) suitable for energy storage applications. BMIM AOT:TFTFE mixtures at 2:1, 1:1, and 1:2 weight ratios were investigated using rheological measurements, conductivity analysis, cyclic voltammetry, electrochemical impedance spectroscopy, small- and wide-angle X-ray scattering (S/WAXS), and atomic force microscopy (AFM). 1,1,2,2-Tetrafluoroethyl-2,2,2-trifluoroethyl ether addition at 1:1 weight ratio reduced BMIM AOT viscosity by 99 % and increased conductivity by over one order of magnitude while maintaining electrochemical stability (>4 V). S/WAXS revealed preservation of characteristic sponge-like nanostructures in the bulk for all BMIM AOT:TFTFE mixtures investigated. Differential capacitance measurements showed enhanced charge storage capabilities, with maximum performance at 1:1 ratio due to improved ion mobility. AFM showed that 1,1,2,2-tetrafluoroethyl-2,2,2-trifluoroethyl ether enhances solvophobic segregation at neutral interfaces but reduces interfacial nanostructures at charged surfaces. These findings reveal that SAIL-based LCILs achieve optimal balance between low viscosity and stable nanostructures, making them promising electrolytes for energy storage devices requiring fast charge-discharge rates and electrochemical stability.[1]
Stable Sodium-Sulfur Batteries
Room-temperature sodium-sulfur (RT Na-S) batteries are garnering interest owing to their high theoretical energy density and low cost. However, the notorious shuttle behavior of sodium polysulfides (NaPS) and uncontrollable dendrite growth lead to the poor cycle stability of RT Na-S cells. In this work, we report the use of 1,2-dimethoxypropane (DMP) and 1,1,2,2-tetrafluoroethyl-2,2,2-trifluoroethyl ether (TFTFE) as inner solvent and outer diluent, respectively, in a localized high-concentration electrolyte system. Impressively, the asymmetric DMP as the inner solvent, introduced to replace the conventional solvent 1,2-dimethoxyethane (DME), shields NaPS effectively from incorporation into the inner solvation structure due to the extra methyl groups in the molecular structure. Furthermore, the 1,1,2,2-tetrafluoroethyl-2,2,2-trifluoroethyl ether diluent, which contains electron-withdrawing perfluoro segments (-CF3- and -CF2-), exhibits significantly low solvation power. Consequently, the outer sheath 1,1,2,2-tetrafluoroethyl-2,2,2-trifluoroethyl ether diluent further minimizes NaPS dissolution, thereby enhancing the cycle stability. This inner-outer sheath synergistic effect leads to the formation of highly effective cathode-electrolyte interphase (CEI) and solid-electrolyte interphase (SEI) layers simultaneously, significantly alleviating the shuttle effect and reducing the side reactions between NaPS and sodium metal. Remarkably, the Na-S cells with the designed electrolyte present long-cycling reversibility with 530 mAh g-1 over 600 cycles at a C/2 rate and a low capacity decay rate of 0.077% per cycle. This study provides a profound understanding of the electrolyte structure involving NaPS and offers a firm basis for the rational design of electrolytes for rechargeable metal-sulfur battery systems.[2]
High-Performance Lithium-Ion Cells at High Voltage
An experimental investigation is conducted to identify the optimal blend of fluoroethylene carbonate (FEC), 3,3,3-trifluoropropylene carbonate (TFEC), and various fluorinated ethers, including 1,1,2,2-tetrafluoroethyl-2,2,2-trifluoroethyl ether (HFE), 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE), and bis(2,2,2-trifluoroethyl) ether (BTE), to enhance the performances of lithium-ion cells at high voltage. The cell incorporating TTE exhibits a significantly superior capacity for retention after long-term cycling at 4.5 V, which might be attributed to the improved kinetics of lithium ions and the generation of a thin, reliable, and inorganic-rich electrode-electrolyte interface. This enhancement facilitates greater lithium ion mobility within the cell, while effectively suppressing active lithium loss and side reactions between the electrodes and electrolytes at elevated voltages. Furthermore, the cell with TTE demonstrates a superior rate capability and high-temperature performance. As a result of the inherent safety characteristics of these all-fluorinated electrolytes, cells using these formulations show excellent safety properties under typical abuse scenarios. Except at elevated temperatures, none of the cells undergo thermal runaway when subjected to mechanical or electrical abuse, and there are minimal differences in safety performance across the different formulations. Considering electrochemical performance, safety, and cost factors, it can be concluded that TTE might be more optimal to cooperate with FEC and TFEC for high-performance high-voltage cells.[3]
References
[1]Li H, Gorenskaia E, Morice R, et al. Electrochemistry and structure of locally concentrated surface-active ionic liquids. J Colloid Interface Sci. 2026;702(Pt 2):138968. doi:10.1016/j.jcis.2025.138968
[2]Yao W, Pai MH, Manthiram A. Inner-Outer Sheath Synergistic Shielding of Polysulfides in Asymmetric Solvent-Based Electrolytes for Stable Sodium-Sulfur Batteries. J Am Chem Soc. 2025;147(14):12061-12074. doi:10.1021/jacs.4c18374
[3]Sheng Y, Liu B, He J, Zhi M, Ouyang D. Optimal Blend Between Fluorinated Esters and Fluorinated Ether for High-Performance Lithium-Ion Cells at High Voltage. Materials (Basel). 2025;18(2):274. Published 2025 Jan 9. doi:10.3390/ma18020274
Lastest Price from 1,1,2,2-Tetrafluoroethyl 2,2,2-trifluoroethyl ether manufacturers
US $0.00/KG2023-05-03
- CAS:
- 406-78-0
- Min. Order:
- 1KG
- Purity:
- 99.5%
- Supply Ability:
- 1000
US $0.00/kg2022-09-20
- CAS:
- 406-78-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg