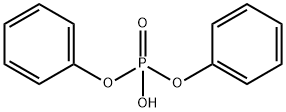
Diphenyl Phosphate: Toxicity, Bioaccumulation & Association with Thyroid Function
Diphenyl phosphate is an aryl phosphate and a metabolite of some flame retardants. Diphenyl phosphate can be used as an organic catalyst for the ring-opening polymerization (ROP) of renewable 5-alkyl δ-lactones. In combination with zinc iodide, it forms a novel initiating system for the living cationic polymerization of isobutyl vinyl ether.

Diphenyl Phosphate-Induced Toxicity During Embryonic Development
Diphenyl phosphate (DPHP) is an aryl phosphate ester (APE) used as an industrial catalyst and chemical additive and is the primary metabolite of flame retardant APEs, including triphenyl phosphate (TPHP). TPHP is primarily metabolized to DPHP via cleavage of an ester bond between the phosphate group and one of three benzene rings. Metabolism of TPHP to DPHP is mediated by cytochrome P450 enzymes within the liver and serum. DPHP can also be generated from metabolism of ethylhexyl diphenyl phosphate and resorcinol bis(diphenylphosphate), both of which are used as APE flame retardants and plasticizers. As it is currently unknown whether DPHP affects developing embryos, the objective of this study was to determine the potential for DPHP-induced toxicity during embryonic development. Using zebrafish as a model, we relied on phenotyping, mRNA-sequencing, and hemoglobin quantification in situ to determine the potential impacts of DPHP on cardiac morphogenesis, the embryonic transcriptome, and red blood cell formation, respectively, following embryonic exposure from 24 to 72 h postfertilization (hpf). Finally, we quantified embryonic doses of DPHP and TPHP at 30, 48, and 72 hpf following initiation of Diphenyl phosphate exposure at 24 hpf.[1]
Within this study, we found that exposure to high concentrations of Diphenyl phosphate (1000 μM) blocked cardiac looping and increased SV-BA length in the absence of effects on survival, hatch rate, and gross morphology (body length, pericardial area, and yolk sac area). There is a possibility that, at concentrations higher than 1000 μM, DPHP may adversely affect survival, hatch rate, and/or growth. However, the potency and toxicity of DPHP within developing zebrafish embryos was minimal even at the highest nominal concentration tested (1000 μM). On the basis of a targeted gene expression panel using chicken embryonic hepatocyte cultures, Diphenyl phosphate exposure significantly altered transcripts related to Phase I or II metabolism, and farnesoid X receptor/liver X receptor signaling. However, to our knowledge, no previous studies have evaluated the potential effect of DPHP on the transcriptome within any model system. To our knowledge, this is one of the first studies to investigate the potential of DPHP-induced effects on early embryonic development. Collectively, our data suggest that high concentrations of Diphenyl phosphate (1000 μM) induce cardiotoxicity and hemotoxicity during embryonic development within zebrafish. Interestingly, adverse effects of DPHP on the embryonic transcriptome at 30 and 48 hpf preceded detectable, downstream effects on cardiac development and red blood cell formation phenotypes that may have been driven by mitochondrial dysfunction earlier in development.
In Vivo Characterization of the Toxicological Properties of Diphenyl phosphate
Diphenyl phosphate (DPhP) has been used as a main biomarker for assessing exposure to aryl phosphate esters (APEs), especially triphenyl phosphate (TPhP), a molecule suspected of presenting human health hazards. However, DPhP can be produced from degradation of several APEs. Moreover, DPhP itself is largely present in the environment worldwide, either owing to its spontaneous/microorganism production from known APEs, or to its direct use in industry. To validate our choice of using Diphenyl phosphate rather than TPhP or another APE in our toxicity study, we first analyzed DPhP concentrations in blood of mice treated with various doses of both molecules via different routes of exposure. We hypothesized that humans are more likely to be continuously/chronically exposed to TPhP and Diphenyl phosphate owing to the presence of TPhP in the air and dust, rather than temporarily/acutely exposed through nutrition. We have thus analyzed the effects of continuous absorption of these two molecules through drinking water via kinetic measurement of their transformation in mice (bioaccumulation and tissue distribution), in comparison with other acute modes of administration such as oral gavage or tail-vein injection. We then present the data reporting the bioaccumulation and distribution of these molecules in mice.[2]
Only few studies have been conducted to directly test the effect of Diphenyl phosphate, the most common APE derivative in human samples. Our results showed that at least in mice, DPhP levels in biological fluids are unlikely to represent a surrogate of direct APE ingestion. We demonstrated that even through direct and acute exposure by IV or oral gavage, only a minor fraction of a parent APE such as TPhP were converted in vivo into DPhP. Moreover, during an overnight exposure to low TPhP concentrations in drinking water, conversion of TPhP into DPhP could no longer be measured. In vivo Diphenyl phosphate may thus be a surrogate of spontaneous degradation of APEs in the environment, suggesting that experimental procedures revolving around DPhP are likely more relevant for assessing APE toxicity. Our results raised many questions on the use, the safety and the presence in the environment of APEs, most of which may expose humans to Diphenyl phosphate. However, the known function of these factors and their association with the metabolic syndromes should constitute a sufficiently strong risk factor to measure more precisely the health hazards associated with their presence in the environment, by taking into consideration diet in future epidemiological studies.
Associations Between Urinary Diphenyl Phosphate and Thyroid Function
Organophosphate flame retardants are widely used in commercial and consumer products. Their use has increased over the past decade, in part due to the phase out of certain polybrominated diphenyl ethers (PBDEs) such as PentaBDE. Methods have been developed to measure diphenyl phosphate (DPHP), a urinary metabolite of TPHP. However, most of these studies had small sample sizes with short sampling periods that may not capture potential long term or seasonal variability. The current study aims to characterize urinary DPHP concentrations in a population of U.S. adults, assess intra-individual variability of repeated DPHP measures, and investigate the association between adult DPHP concentrations and thyroid function. We measured urinary Diphenyl phosphate and serum thyroid hormones in repeated samples from a group of 51 male and female office workers over a one-year period as part of a study investigating exposure patterns and health effects of flame retardant chemicals in the Boston, MA area. We hypothesized that there would be high intra-individual variability in urinary DPHP concentrations over the study period and that DPHP concentrations would be associated with altered serum thyroid hormone levels. We explored whether factors including PBDEs, urinary iodine, age, or sex might modify associations between DPHP and thyroid hormones. Additionally, we characterized urinary DPHP concentrations at a single time point in a subset of the study participants' children.[3]
We detected DPHP in nearly all urine samples from the FlaRE study population. Concentrations were higher in women compared to men, higher in samples collected later in the day, and varied significantly within individuals across sampling rounds. We found a positive association between SG-corrected Diphenyl phosphate concentrations and TT4 levels among women. We found evidence that urinary DPHP concentrations are higher in women and may be associated with increased TT4 levels, especially among women. DPHP was not strongly associated with TSH or FT4. As TPHP is almost ubiquitously detected in urine and this is only the second study to assess these relationships, further studies in larger, mixed sex populations are needed to elucidate the relationship between urinary Diphenyl phosphate concentrations and thyroid function.
References
[1]Mitchell CA, Reddam A, Dasgupta S, Zhang S, Stapleton HM, Volz DC. Diphenyl Phosphate-Induced Toxicity During Embryonic Development. Environ Sci Technol. 2019 Apr 2;53(7):3908-3916. doi: 10.1021/acs.est.8b07238. Epub 2019 Mar 20. PMID: 30864794; PMCID: PMC6445678.
[2]Selmi-Ruby S, Marín-Sáez J, Fildier A, Buleté A, Abdallah M, Garcia J, Deverchère J, Spinner L, Giroud B, Ibanez S, Granjon T, Bardel C, Puisieux A, Fervers B, Vulliet E, Payen L, Vigneron AM. In Vivo Characterization of the Toxicological Properties of DPhP, One of the Main Degradation Products of Aryl Phosphate Esters. Environ Health Perspect. 2020 Dec;128(12):127006. doi: 10.1289/EHP6826. Epub 2020 Dec 9. PMID: 33296241; PMCID: PMC7725437.
[3]Preston EV, McClean MD, Claus Henn B, Stapleton HM, Braverman LE, Pearce EN, Makey CM, Webster TF. Associations between urinary diphenyl phosphate and thyroid function. Environ Int. 2017 Apr;101:158-164. doi: 10.1016/j.envint.2017.01.020. Epub 2017 Feb 3. PMID: 28162782; PMCID: PMC5348264.
You may like
See also
Lastest Price from Diphenyl phosphate manufacturers

US $100.00-75.00/kg2025-04-21
- CAS:
- 838-85-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000

US $79.00-38.00/kg2025-04-21
- CAS:
- 838-85-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton