4F-MPH: Novel Psychoactive Substance
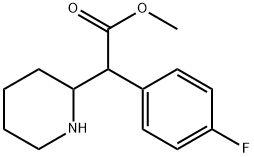
4F-MPH is a synthetic molecule of the substituted phenethylamine and substituted phenidate classes, and a fluorinated analog of methylphenidate. It contains a phenethylamine core featuring a phenyl ring bound to an amino -NH2 group through an ethyl chain. It is structurally similar to amphetamine, featuring a substitution at Rα which is incorporated into a piperidine ring ending at the terminal amine of the phenethylamine chain. Additionally, it contains a methyl acetate bound to Rβ or its structure. 4F-MPH is structurally identical to methylphenidate with the exception of a single fluorine atom bound to the four position on the phenethylamine core. With respect to nomenclature, the methyl- in methylphenidate regards the side chain of one carbon atom, while phen- indicates the phenyl ring. Id- is contracted from a piperidine ring, and -ate indicates the acetate group containing the oxygen atoms. Like its parent molecule, 4F-MPH is a chiral compound, presumably produced as a racemic mixture.

Analytical characterization and pharmacological evaluation of 4F-MPH
Methylphenidate (methyl-2-phenyl-2-(piperidin-2-yl)acetate; MPH; Ritalin) is a substituted phenethylamine that was first synthesized in 1944 and subsequently recognized as a psychostimulant. 4-Fluoromethylphenidate (4F-MPH) is a methylphenidate analog that was developed within the pharmaceutical setting. However, in November 2015, it was first notified by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Early Warning System following its detection on the recreational market. Previous in vitro studies indicated that the addition of a fluorine atom to the para-position of the phenyl ring in the methylphenidate parent structure led to slight increases in potency related to displacement of [3H]WIN-35,428 and [3H]dopamine uptake inhibition. This study describes the analytical characterization of two powdered samples and a set of tablets of 4-fluoromethylphenidate that were obtained from the same vendor based in the United Kingdom in 2015. The study was triggered following the receipt of two powdered 4F-MPH samples from an online retailer. Various chromatographic, spectroscopic and mass spectrometric analysis methods were employed, followed by x-ray crystal structure analysis. It was hypothesized that the distinct forms of 4F-MPH encountered in these products would result in different pharmacological properties similar to what has been reported for MPH, thus, potentially resulting in different effects in consumers.[1]
Manufacturers and entrepreneurs dedicated to the commercial exploration of ‘research chemicals’ destined for recreational drug markets, are utilizing the scientific and patent literature to generate ideas for launching new compounds. Since methylphenidate and a series of its analogs have been well documented in the literature, e.g., it was not surprising to see that several methylphenidate-based NPS have also been encountered on the ‘research chemicals’ market. The analytical characterization of two 4F-MPH powders obtained from the same vendor of ‘research chemicals’ revealed the presence of (±)-threo isomers in one and a mixture of (±)-threo / (±)-erythro-4F-MPH racemates in the other, which suggested a failure to isolate the (±)-threo-racemate that may have formed during the synthesis procedure of the main (±)-threo product. Various chromatographic, spectroscopic and mass spectrometric platforms were employed followed by x-ray crystal structure analysis. The significantly reduced potency of (±)-erythro-4F-MPH was consistent with (±)-erythro-MPH and other (±)-erythro-MPH analogs reported in the literature. These findings suggest that the psychostimulant properties of (±)-threo-4F-MPH might be more potent in humans than MPH. Since the biological activity resides in the (±)-threo form, it is anticipated that other MPH-derived ‘research chemicals’ on the market might also display this configuration.
4-Fluoromethylphenidate as a Previously Unreported Novel Psychoactive Substance
Since the emergence of synthetic cathinones in 2012, post-mortem toxicological casework in Miami-Dade County, Florida has been rampant with the presence of novel psychoactive substances (NPSs). These compounds, including synthetic cathinones, synthetic cannabinoids, designer hallucinogens and synthetic opioids, consistently evolve to evade restrictions from both foreign and domestic drug enforcement agencies. As the number of NPS increase, so does the demand for accurate and reliable identifications of these compounds in complex biological matrices. Recently, a new class of NPS similar in structure to methylphenidate (MPH) was identified in a case received from Collier County, Florida. The substance identified and confirmed by the laboratory was (±)-threo-4-fluoromethylphenidate (4F-MPH). The Miami-Dade County Medical Examiner (MDME) Toxicology Laboratory first encountered it in 2016 in the form of drug paraphernalia. The case containing the drug paraphernalia was a delayed death due to a multiple drug toxicity, and among the several exhibits of paraphernalia that were collected at the scene, an orange round pill with an ‘A’ stamped onto one side was analyzed in the laboratory and positive for 4F-MPH. To confirm the presence of this new NPS, a reference standard was ordered and added to all in-house screening libraries. While 4F-MPH was confirmed in the paraphernalia, it was never detected in any biological specimens in the decedent.[2]
This case report describes the sudden death of a 25-year-old male who consumed multiple drugs including 4F-MPH. Based on the toxicology and medical examiner’s findings, the decedent likely succumbed to an acute overdose of heroin; however, the effects of methamphetamine, 3-MeO-PCP, codeine and 4F-MPH cannot be overlooked as contributing to death. The majority of fatal cases investigated by the MDME that involve NPS are poly-drug intoxications, where one or more of the drugs present in the decedent contribute solely to the cause of death; however, in many other cases the multiple drugs present in combination contributed to the death. Consequently, understanding the toxicological significance of a single new NPS is difficult at best; however, the contributions of these NPS compounds cannot be ignored. The flood of newly synthesized drugs over the past 6 years has covered a broad range of substances that have included stimulants, depressants and hallucinogens. Many of the most common substances have stemmed from the structural foundations of cathinones, cannabinoids, benzodiazepines and fentanyl. However, more fringe substances have emerged that are not common such as 4F-MPH. As a result, it is likely that additional NPS will become available furthering the need for laboratories to increase their scope of testing.
Analytically Confirmed Intoxication by 4-Fluoromethylphenidate
4-Fluoromethylphenidate is an halogenated derivative of methylphenidate (MPH), a re-uptake inhibitor for dopamine and norepinephrine used for the treatment of attention deficit hyperactivity disorders. In the last few years, several compounds structurally related to MPH have been marked as new psychoactive substances (NPS) with stimulating and euphoric effects similar to the parent drug, but with more dopaminergic activity. This report represents the first case of an analytically confirmed non-fatal intoxication by 4F-MPH. A 26-year-old female was admitted to the emergency department with neuropsychiatric and cardiologic symptoms that lasted for a week, during which she sniffed a powder named 4F-MPH acquired as entactogen on the Internet. The patient required sedation with intravenous diazepam and was discharged two days later with a prescription of promazine and quetiapine. The seized product was analytically characterized by gas chromatography-mass spectrometry, liquid chromatography high-resolution mass spectrometry and nuclear magnetic resonance.[3]
Analyses confirmed the composition of the product as a 4F-MPH diastereomeric (±)-threo and (±)-erythro mixture, with a large preponderance of the active (±)-threo isomer. The blood and urine concentration of (±)-threo 4F-MPH were 32 ng/mL and 827 ng/mL, respectively. Analyses for classic drugs (opiates, methadone, cocaine, cannabis metabolites, amphetamines, ecstasy and LSD), ethanol, qualitative full screen by gas chromatography-mass spectrometry and targeted analysis for 50 NPS by liquid chromatography-tandem mass spectrometry tested negative; comorbidities were excluded, too.
References
[1]McLaughlin G, Morris N, Kavanagh PV, Power JD, Dowling G, Twamley B, O'Brien J, Hessman G, Murphy B, Walther D, Partilla JS, Baumann MH, Brandt SD. Analytical characterization and pharmacological evaluation of the new psychoactive substance 4-fluoromethylphenidate (4F-MPH) and differentiation between the (±)-threo and (±)-erythro diastereomers. Drug Test Anal. 2017 Mar;9(3):347-357. doi: 10.1002/dta.2167. Epub 2017 Mar 7. PMID: 28103426; PMCID: PMC5378611.
[2]Shoff, Elisa N et al. “4-Fluoromethylphenidate: Fatal Intoxication Involving a Previously Unreported Novel Psychoactive Substance in the USA.” Journal of analytical toxicology vol. 43,8 (2019): 666-672. doi:10.1093/jat/bkz061
[3]Papa, Pietro et al. “Analytically Confirmed Intoxication by 4-Fluoromethylphenidate, an Analog of Methylphenidate.” Journal of analytical toxicology vol. 43,5 (2019): e1-e7. doi:10.1093/jat/bkz001
You may like
See also
Lastest Price from 4F-MPH manufacturers

US $15.00/KG2024-01-19
- CAS:
- 1354631-33-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T

US $30.00/kg2023-09-20
- CAS:
- 1354631-33-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons