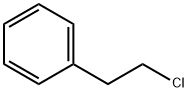
Phenethyl Chloride: Pyrolysis Mechanism in PVC Recycling
Phenethyl chloride is an aromatic halogenated hydrocarbon containing a benzene ring and a chloroethyl group. At ambient temperature, it exists as a colourless liquid with a boiling point of approximately 190–192°C. It is poorly soluble in water but readily soluble in organic solvents. It may be synthesised by reacting phenethyl alcohol with a chlorinating agent such as SOCl₂. As a key intermediate in organic synthesis, Phenethyl chloride finds extensive application in the production of pharmaceuticals, fragrances, dyes, and fine chemicals. Caution is advised due to its irritant properties, necessitating appropriate protective measures during handling.

Pyrolysis Reactions of Phenethyl chloride
Polyvinyl chloride (PVC) is a synthetic thermoplastic material commonly used in building materials. In recent years, it has ranked among the top three major polymers for global plastics production − and among the top five in market share and industrial applications. Recent research, however, documents many unfavorable health − and environmental − outcomes associated with its use and waste management. The pyrolysis of PVC primarily releases HCl, aromatics, hydrocarbons, and chlorinated hydrocarbons. − The chlorinated hydrocarbons are of special concern in the chemical recycling of PVC because they are toxic, corrosive, and have limited industrial value. One common example of a chlorinated hydrocarbon derived from the pyrolysis of PVC is Phenethyl chloride. There has been much research focusing on thermal decomposition pathways for PVC, but limited work has focused on the thermal decomposition pathway of the individual chlorinated hydrocarbons generated during that process. The simplest aromatic chlorides, chlorobenzene and benzyl chloride, have been studied by pyrolytic techniques, so the unstudied and slightly more complex Phenethyl chloride is a natural choice for a pyrolysis study. Understanding the high-temperature reactions of Phenethyl chloride will facilitate the development of a pyrolysis mechanism and also the development of methods to manage undesired byproducts of the PVC chemical recycling process.[1]
HCl elimination reactions are common in both the pyrolysis and photolysis of many chlorinated hydrocarbons. A four-centered HCl elimination reaction of Phenethyl chloride should lead to the formation of styrene. To determine whether styrene was indeed a pyrolysis product, a commercial sample of styrene was obtained, and an argon-matrix FTIR spectrum was recorded. The unheated sample acted as a benchmark to compare whether the bands for styrene matched any peaks in the spectrum from the heated sample of Phenethyl chloride. The evidence for styrene and HCl production from Phenethyl chloride observed here is quite convincing, but the eventual fate of styrene is an interesting point to consider. Styrene pyrolysis is known to lead to polycyclic aromatic hydrocarbon production via bimolecular reactions, as observed for high-pressure samples at 1100–1730 K and the liquid at 500–900 °C. A low-pressure (∼10 mTorr) flow reactor study of styrene at 1180–1350 K detected benzene and acetylene and suggested a unimolecular pathway. These latter conditions are more relevant to the experiments conducted here and suggest that benzene and acetylene observed in the pyrolysis of Phenethyl chloride could be produced by reactions of styrene.
Here, there is no evidence of the production of chloromethyl radicals or benzyl radicals following the pyrolysis of Phenethyl chloride. Another helpful analogue to consider for insight into the pyrolysis of Phenethyl chloride is 1-bromo-1-phenylethane. During pyrolysis, 1-bromo-1-phenylethane decomposes via HBr elimination to make styrene. To better understand the mechanism of the formation of styrene and then phenylacetylene, computational chemistry was used to map the possible pathways to these products. We show the B3LYP-optimized geometries of the anti- and gauche-conformers of Phenethyl chloride, styrene, phenylacetylene, intermediates, and transition states along those paths.The pyrolysis products of Phenethyl chloride in argon were identified by matrix-isolation FTIR spectroscopy. It was observed that the pyrolysis products of Phenethyl chloride were HCl, styrene, phenylacetylene, benzene, vinylacetylene, acetylene, propyne, ethylene, propargyl radical, and several HCl-based complexes. Styrene was a predominant product likely produced through a four-centered elimination mechanism. Other products, particularly phenylacetylene, benzene, and acetylene, may be due to the secondary reactions of styrene. These results provide a good foundation for mechanism development and have potential applications in the management of chlorinated hydrocarbons generated during PVC chemical recycling.
References
[1] Jarrell, Mia et al. “Pyrolysis Reactions of (2-Chloroethyl)benzene.” The journal of physical chemistry. A vol. 129,41 (2025): 9521-9528. doi:10.1021/acs.jpca.5c0372
You may like
Lastest Price from Phenethyl chloride manufacturers

US $0.00/KG2025-04-21
- CAS:
- 622-24-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-03-07
- CAS:
- 622-24-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons