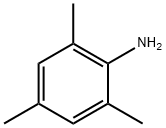
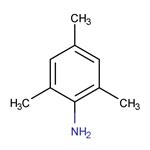
2,4,6-trimethylaniline:Uses,Toxicity and Synthesis
2,4,6-trimethylaniline is a kind of intermediate of acid mordant dye, is widely used in dyestuffs industries.

Uses
2,4,6-Trimethylaniline is used to produce commercial quantities of Acid Blue 129 dye, which is used in histochemistry studies.
Occurence
2,4,6-Trimethylaniline has been detected among the volatile compounds in the smoke distillates from one type of tobacco (Latakia); thus it can be present in tobacco smoke. 2,4,6-Trimethylaniline is produced by microbes and mammals, including humans, during the metabolism of certain azo dyes. The general public may be exposed through tobacco smoke. Workers can be exposed in manufacturing processes that use the compound.
Formation of active metabolites
2,4,6-Trimethylaniline can be metabolited to N-hydroxylated metabolites, including 3,5-dimethyl, 2- and 4-amino benzoic acids, 2,6-dimethyl quinone and hydroquinone, and conjugates (Lindstrom et al., 1969). Formation of N-hydroxylated metabolites and conjugates is considered to be an important step in the activation of various carcinogenic aromatic amines.
Toxicity
2,4,6-Trimethylaniline has acute toxicity, it is harmful if swallowed or in contact with skin.
2,4,6-Trimethylaniline induced methemoglobinemia and anaemia in rats. Both syndromes are indicators of the formation of N hydroxylated metabolites: IARC (1882).
Synthesis
The advantage of the radical process, that substitution of nucleofuges by NH2− on an aromatic ring takes place without cine substitution (by the aryne mechanism), was clearly seen in the first aromatic Srn1 reactions recognized. The same regiochemical advantage is seen in the potassium-stimulated conversion of o-haloanisoles predominantly into o-anisidine rather than the m-isomer (the sole product of the aryne process). Also, unlike substitutions proceeding by the aryne mechanism, reactions readily take place when both ortho positions are blocked, and 2-bromomesitylene reacted with NH2−under photostimulation to give 2,4,6-trimethylaniline in 70% yield.[1]
Reference
[1] NORRIS R K. Nucleophilic Coupling with Aryl Radicals[C]. 1900: 0. DOI:10.1016/B978-0-08-052349-1.00100-1.
Lastest Price from 2,4,6-Trimethylaniline manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 88-05-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $120.00/kg2025-04-15
- CAS:
- 88-05-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton