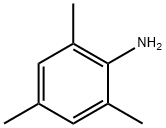
Chemical Properties
clear yellow to brown liquid. Insoluble in water, soluble in organic solvents such as ethanol and ether.
Uses
2,4,6-Trimethylaniline is useful for the preparation of Grubbs' catalyst. It is used as a precursor to dyes. It undergoes condensation reaction with glyoxal to prepare glyoxal-bis(mesitylimine). It is also involved in the preparation of 1,3-diketimines ligands by condensation with 1,3-diketones. Further, it is used for the production of Acid Blue 129 dye, which is useful in histochemistry studies. In addition to this, it serves as a building block to various bulky ligands.
Preparation
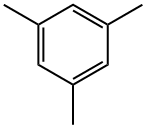
2,4,6-Trimethylaniline is prepared by selective mononitration of mesitylene, avoiding oxidation of the methyl groups.
Mix sulfuric acid and nitric acid into mixed acid, nitrify with mesitylene at atmospheric pressure below 10°C for 4 hours, stand for stratification, and let off the waste acid. The obtained 2,4,6-trimethylnitrobenzene, iron powder, hydrochloric acid and water were prepared in a ratio of 1:3.74:0.9:2.22 (mol ratio) for reduction reaction at 100-105°C for 8h. Crude 2,4,6-Trimethylaniline was then distilled off from the reduced material and purified by distillation.
Definition
ChEBI: 2,4,6-Trimethylaniline is a substituted aniline.
General Description
2,4,6-Trimethylaniline is a clear liquid. easily turns light brown when exposed to air and light. It is an intermediate for dyes, organic pigments and pesticides.
Reactivity Profile
2,4,6-Trimethylaniline neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Health Hazard
2,4,6-Trimethylaniline is moderately toxic orally. It is also considered highly toxic by unspecified routes. It is a skin and eye irritant. Suspect occupational carcinogen. (Non-Specific -- Aromatic Amines) The danger of acute poisoning is represented by methemoglobinemia leading to adverse effects on the red cells. A number of the amines may act as skin sensitizers.
Fire Hazard
When heated to decomposition, 2,4,6-Trimethylaniline emits toxic fumes of nitrogen oxides. Avoid decomposing heat.
Safety Profile
Poison by inhalation.
Moderately toxic by ingestion. A skin and
severe eye irritant. Questionable carcinogen
with experimental carcinogenic data.
Mutation data reported. When heated to
decomposition it emits toxic fumes of NOx.
Potential Exposure
Used on small scale in organic
synthesis.
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.