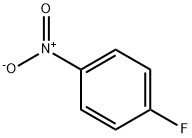
4-Fluoronitrobenzene: Nucleophilic Aromatic Substitution & Fine Chemical Synthesis
4-Fluoronitrobenzene is an important aromatic organic intermediate with the molecular formula C₆H₄FNO₂, featuring a fluorine atom and nitro group in the para-position on the benzene ring. It serves as a prototypical nucleophilic aromatic substitution substrate, utilised in synthesising diverse fine chemicals including pharmaceuticals, dyes, and pesticides. Furthermore, 4-Fluoronitrobenzene frequently functions as a model compound for investigating reaction mechanisms (such as substitution reactions with amines and phenols). Its distinctive electronic effects—the synergistic interaction between the electron-withdrawing nature of the nitro group and the electron-donating character of fluorine—endow it with extensive application value in organic synthesis.

Biphasic nucleophilic aromatic substitution using a microreactor
Biphasic organic reactions using a phase transfer catalyst are advantageous due to the fact that they use common inexpensive solvents and simple separation procedures. As a result, many such chemical processes have been developed on an industrial scale. However, in many biphasic reactions, the rate-determining step is the mass transfer process between the two phases, and the reaction rate is often slower than that of a comparable homogeneous reaction. Our group also reported that the nucleophilic aromatic substitution of 4-fluoronitrobenzene by ethanol proceeded under high temperature and pressure conditions. Although the target compound (4-ethoxy-nitrobenzene) was obtained in a good yield under slug flow conditions, it was difficult to control the formation of 4-hydroxy-nitrobenzene as a byproduct under high temperature conditions. Thus, to realize an efficient synthetic method, we planned to investigate the reaction under bubbly flow conditions, in which droplet size was further reduced from those associated with slug flow conditions. Thus, we herein report our examination of the aromatic nucleophilic substitution reaction of 4-fluoronitrobenzene under droplet formation conditions, with the aim of achieving substitution under mild reaction conditions with short reaction times.[1]
To demonstrate a potential application of this reaction, we synthesized the Fab I inhibitor 7, which is a physiologically active the substance. More specifically, 2-(2-methoxy-4 methylphenoxy)-nitrobenzene 7 was synthesized in 92% yield by nucleophilic aromatic substitution of 4-fluoronitrobenzene with o-methoxy-p-methylphenol 6 under flow conditions using the packed bed mixer (Scheme 2). This was achieved by feeding a solution of 0.2 M 2-fluoronitrobenzene into the mixer with a 2 N NaOH aq. solution containing 0.22 M 6 to give product 7 after a reaction time of only 4 min at 100 °C. In conclusion, we carried out a biphasic reaction under flow conditions using a packed bed mixer. We revealed that the packed bed mixer assisted the formation of fine droplets from biphasic solvents, and the desired nucleophilic aromatic substitution reaction proceeded efficiently under the flow conditions. The biphasic nucleophilic aromatic substitution of 4-fluoronitrobenzene was carried out using a microreactor. To realize an efficient reaction process, we performed microflow reactions under droplet formation conditions to give the substituted products in excellent yields. The solvent effect was also investigated, and it was found that the use of diisobutyl ketone as an organic phase greatly accelerated the reaction.
Nucleophilic Substitution of 4-Fluoronitrobenzene withPolyvinylamine
Poly(vinylformamide-co-vinylamine)s (PVFA-co-PVAm) are commercially available polyelectrolytes for widespread applications. PVFA-co-PVAm with well-defined molecular masses and controlled amounts ofPVAm segments can be synthesized from polyvinylformamide by base- or acid-catalyzed hydrolysis reactions. In this Communication we for the first time report the nucleophilic substitution of activated aromatic fluoro-substituted compounds, e. g., 4-fluoronitrobenzene and others, with PVAm (M—n = 60 000 g N mol – 1 ) in water, mediated by cyclodextrins. The nucleophilic substitution of activated aromatics with primary amines is a well-established synthetic procedure utilizing for example Sanger’s reagent or other aromatics containing electron-withdrawing groups in conjugation to the fluorine substituent, which serves as a good leaving group.[2]
In order to check the synthetic potential of the method, various aromatic fluoro compounds were tested, differing significantly in their reactivity towards nucleophiles. 4-Fluoronitrobenzene (4-FNB) was chosen as a model compound for optimizing the reaction conditions with PVAm in water, because of its adjustable reactivity due to the good solubility of its corresponding b-CD or b-DMCD complex in water. For the work reported here, the4-FNB/b-DMCD complex was applied. Polyvinylamine can be readily functionalized by means of a nucleophilic substitution reaction with 4-fluoro-substituted aromatic compounds having a substituent with negative mesomeric effect in ortho and/or para position. PVAm is soluble in water only in which aromatic fluoro compounds are usually sparingly soluble. However, the solubility of aromatic compounds in water can be mediated in particular by cyclodextrin derivatives. This first report on the nucleophilic substitution reaction of 4-fluoronitrobenzene with PVAm provides an elegant method towards the preparation of chromophoric water-soluble polymers by means of a one-pot procedure.
References
[1]Mori, H., Nishiyama, Y., Fujii, A., Saito, A., Torikai, H., Hanasaka, T., & Koishi, H. (2021). Biphasic nucleophilic aromatic substitution using a microreactor under droplet formation conditions. Reaction Chemistry & Engineering, 6(4), 1012-1017.
[2]Roth, I., & Spange, S. (2001). Nucleophilic substitution of 4-fluoronitrobenzene with polyvinylamine in water mediated by cyclodextrins. Macromolecular Rapid Communications, 22(15), 1288-1291.
You may like
Lastest Price from 4-Fluoronitrobenzene manufacturers

US $0.00/KG2025-04-21
- CAS:
- 350-46-9
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 350-46-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt