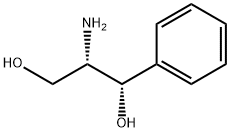
Synthesis and Chemical Applications of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol
(1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol is a chiral amino alcohol compound characterized by its significant polarity and distinctive physiological activity, which exists as a white to yellow solid powder under ambient conditions and, while being poorly soluble in water and diethyl ether, demonstrates good solubility in alcoholic organic solvents. Studies have reported that (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol possesses certain physiological toxicity, with researchers specifically documenting its cytotoxicity to Rat Hepatoma-derived Fa32 cells.
Synthesis
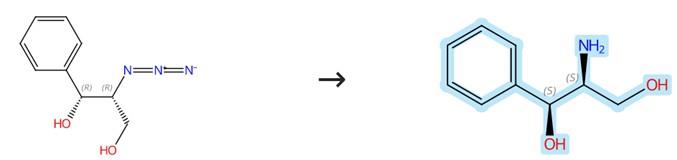
Figure 1: Synthesis of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol
At room temperature, add 20% Pd(OH)2/C (50 mg) to a stirred solution of the azidoalcohol (1.5 g, 7.7 mmol) in methanol (40 ml) while maintaining a hydrogen atmosphere using a hydrogen balloon. Monitor the reaction progress by thin-layer chromatography (TLC) and upon completion, filter the mixture over a celite pad. The resulting filtrate is then concentrated under reduced pressure to afford the crude aminodiol product (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol. [1]
Condensation reaction
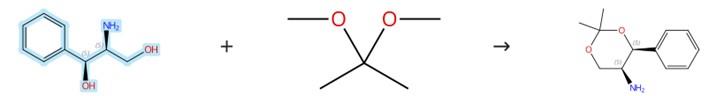
Figure 2: Condensation Reaction of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol
To a solution of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol (500 mg, 3.0 mmol) in MeOH (5 mL), methyl formate (0.2 mL, 3.3 mmol) and NaOMe (16 mg, 0.3 mmol) were added and the resulting mixture was stirred at room temperature for 3.5 hours. After removal of the solvent under reduced pressure, the residue was dissolved in acetone (25 mL) followed by the addition of camphorsulfonic acid (70 mg, 0.3 mmol) and 2,2-dimethoxypropane (3.7 mL, 30 mmol) with subsequent stirring at ambient temperature for 4 hours. The solvents were then evaporated under vacuum and the resulting oily material was redissolved in EtOAc (30 mL), which was then washed with saturated sodium bicarbonate solution (30 mL), dried over magnesium sulfate, filtered, and concentrated in vacuo. The obtained formamide intermediate was dissolved in hydrazine hydrate (50-60%, 20 mL) and heated under reflux conditions for 3 hours. After cooling to 20°C, the mixture was extracted with ethyl acetate (3 × 20 mL) and the combined organic layers were washed with water (2 × 20 mL), then dried over magnesium sulfate, filtered, and finally concentrated under reduced pressure to yield the product. [2]
Inversion of configuration
2-Amino-1-aryl-1,3-propanediols serve as key intermediates in the synthesis of chloramphenicol-type antibiotics, where only the (1R,2R) isomers exhibit biological activity, in stark contrast to their enantiomer, (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol, which is devoid of such efficacy. Unlike the recently reported enantioselective syntheses of chloramphenicol, the industrial production of these amino diols relies on the optical resolution of a racemic intermediate to yield both enantiomers with identical chemical and optical purity, prompting numerous laboratories to explore applications and chemical transformations for the non-target stereoisomers, yet the conversion of the inactive (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol into the bioactive (1R,2R) configuration remains a pressing practical challenge, which has been addressed through two main strategies: one involving racemization—though this requires a subsequent resolution step—and the other employing a stereoselective transformation that directly converts one enantiomer into the other without racemization, with an additional reported method enabling the inversion of configuration at only a single stereogenic center.
Cytotoxicity
Amino alcohols are commonly employed as emulsifying agents in dry-cleaning soaps, wax removers, cosmetics, paints, and insecticides. Using the neutral red uptake inhibition assay, researchers determined the cytotoxicity of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol in normally cultured, glutathione-depleted, or antioxidant-enriched Fa32 rat hepatoma-derived cells. The individual stereoisomers and the racemic mixture of 1-amino-2-propanol exhibited similar cytotoxicities in normally cultured, vitamin E-treated, and BSO-treated Fa32 cells. The position of the hydroxyl group plays a critical role in the toxicity of the compounds, as demonstrated by the significantly different NI50 values observed for 4-amino-1-butanol and 4-amino-2-butanol, and the presence of an additional phenyl group markedly enhanced the cytotoxicity of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol.
Chemical applications
To date, three distinct synthetic routes have been developed for the transformation of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol, which represents another discarded side product of this category, into (R)-(+)-phenylalaninol. One procedure documented in the patent literature accomplishes the preparation of compound 6 directly from aminodiol 2 in a single step through selective hydrogenolysis of the benzylic hydroxyl group, albeit achieving only a 15% yield. The two alternative methodologies each entail five-step reaction sequences, where the first approach commences with the regioselective chlorination of the secondary hydroxyl group in the dihydrochloride salt of (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol, followed successively by N,O-diacetylation, reductive dehalogenation, and final N,O-deprotection steps, affording the desired product (R)-(+)-phenylalaninol in an overall yield of 14%. The other multistep pathway involves the preparation of a cyclic sulfite intermediate from N-phthaloyl-protected aminodiol and thionyl chloride, which upon treatment with lithium bromide undergoes subsequent reductive debromination and removal of the protecting group to furnish target molecule 6 with a significantly improved overall yield ranging between 19% and 33%. [3]
Reference
[1] Prasad, P. K.; et al, Oxidant controlled regio- and stereodivergent azidohydroxylation of alkenes via I2 catalysis, Chemical Communications, 2015, 51, 10276-10279.
[2] D'Arcy, Tom D.;et al, Organocatalytic Enantioselective Synthesis of Bicyclo[2.2.2]octenones via Oxaziridinium Catalysed ortho-Hydroxylative Phenol Dearomatization, Angewandte Chemie International Edition 2022, 61, e202205278.
[3] Scheers EM, Forsby A, Dierickx PJ. Cytotoxicity of Amino Alcohols to Rat Hepatoma-derived Fa32 Cells. Alternatives to Laboratory Animals. 2002;30:309-312.
You may like
See also
Lastest Price from (1S,2S)-(+)-2-Amino-1-phenyl-1,3-propanediol manufacturers
US $0.00/kg2025-03-03
- CAS:
- 28143-91-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $15.00-10.00/KG2021-07-02
- CAS:
- 28143-91-1
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton