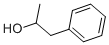Exploring the Chemical Intricacies and Applications of 1-PHENYL-2-PROPANOL
Introduction
1-PHENYL-2-PROPANOL, a compound with a distinctive chemical structure, is not merely a molecule but a cornerstone in the realm of organic chemistry. Its influence extends beyond the confines of laboratories, playing a pivotal role in various industrial applications. In the pharmaceutical sector, it serves as an indispensable precursor for a multitude of drugs, contributing significantly to medical advancements and therapeutic solutions. Its presence in the fragrance industry is equally noteworthy, where its unique olfactory properties are harnessed to craft exquisite scents that enchant and linger. This compound's versatility and utility in these fields underscore its importance and the diverse potential it holds for future applications[1].

Figure 1 Characteristics of 1-PHENYL-2-PROPANOL
Mechanism of Action
1-PHENYL-2-PROPANOL, distinguished by its unique phenyl group attached to the propanol chain, exhibits interesting chemical behavior that is crucial in various chemical syntheses and reactions. The presence of the phenyl group significantly influences the compound's reactivity and interaction with other molecules, particularly in organic synthesis.
In the realm of organic chemistry, 1-PHENYL-2-PROPANOL serves as a vital intermediate. Its ability to undergo oxidation and reduction reactions makes it a versatile agent in synthesizing a plethora of important organic compounds. Moreover, the compound's interaction with different catalysts and its role in facilitating enantioselective reactions are paramount for creating specific isomers of pharmaceutical importance.
Understanding the electronic configuration and steric effects of 1-PHENYL-2-PROPANOL is essential for chemists to manipulate its reactions and predict its behavior in complex chemical environments. The interplay between the phenyl ring and the hydroxyl group offers a fascinating study of intramolecular forces and their impact on the compound's physical and chemical properties.
Applications
1-PHENYL-2-PROPANOL finds its utility in a diverse array of fields, reflecting its versatile chemical nature. One of the primary areas where this compound shows significant value is in the pharmaceutical industry. It acts as a precursor in synthesizing various drugs, particularly those that require a phenylpropanolamine base. Its unique structure enables the formation of compounds that are crucial in producing medications for nasal decongestion and weight loss, among others[2].
In the realm of fragrance and flavor industries, 1-PHENYL-2-PROPANOL is esteemed for its pleasant aromatic properties. It is a key ingredient in the creation of fragrances that mimic natural scents, providing a lasting and stable olfactory experience. This compound's ability to blend with other ingredients without altering their properties makes it a favored choice in the formulation of perfumes and flavorings.
Moreover, 1-PHENYL-2-PROPANOL's role extends to the field of organic chemistry research, where it is used as a standard or reference material in chromatographic analysis and other analytical techniques. Its consistent and predictable behavior under various conditions makes it an ideal compound for scientific studies and experiments.
The broad spectrum of applications of 1-PHENYL-2-PROPANOL underscores its versatility and indispensability in various scientific and industrial fields. Its role in these sectors is a testament to the compound's value in advancing technology and improving the quality of products and services.
Storage Methods
The proper storage of 1-PHENYL-2-PROPANOL is critical to maintain its chemical integrity and ensure its efficacy in various applications. It should be stored in a cool, dry place, away from direct sunlight and heat sources, to prevent any degradation or unwanted reactions. Containers holding the compound need to be tightly sealed to avoid moisture ingress, which can lead to hydrolysis and other detrimental reactions.
In laboratories and industrial settings, it is imperative to store 1-PHENYL-2-PROPANOL in well-ventilated areas to minimize the risk of vapor accumulation, which could lead to respiratory discomfort or other health hazards. Implementing appropriate fire safety measures is crucial, considering the compound's flammable nature.
For long-term storage, inert atmospheres like nitrogen or argon can be used to prevent oxidative degradation. The compatibility of storage materials, such as glass or certain types of plastic, with 1-PHENYL-2-PROPANOL, should be confirmed to avoid any chemical interaction that could compromise the stored compound's purity and effectiveness.
References
[1]Tubergen M J, Lavrich R J, Plusquellic D F, et al. Rotational spectra and conformational structures of 1-phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone[J]. The Journal of Physical Chemistry A, 2006, 110(49): 13188-13194.
[2]Meilahn M K, Statham C N, McManaman J L, et al. Conformational equilibriums in the 1-amino-1-phenyl-2-propanol and 2-amino-1-phenyl-1-propanol systems. III. Nuclear magnetic resonance and infrared studies[J]. The Journal of Organic Chemistry, 1975, 40(24): 3551-3554.
You may like
Related articles And Qustion
See also

US $0.00-0.00/kg2025-09-10
- CAS:
- 14898-87-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20T

US $80.00/KG2023-08-16
- CAS:
- 14898-87-4
- Min. Order:
- 1KG
- Purity:
- >99%
- Supply Ability:
- 20tons