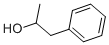The introduction of 1-PHENYL-2-PROPANOL
General description
The 1-PHENYL-2-PROPANOL, with the CAS No: 14898-87-4, is also known as (+/-)-ALPHA-METHYLPHENETHYL ALCOHOL. This chemical’s molecular formula is C9H12O and molecular weight is 136.19. 1-PHENYL-2-PROPANOL can be used as building block for the synthesis of amphetamine hydrochloride, R-Selegiline, cathepsin K and Benzodiazepine. Its structure is as follows:

Figure 1 Structure of 1-PHENYL-2-PROPANOL.
Rotational information of 1-PHENYL-2-PROPANOL
Fifty-nine rotational transitions were measured for 1-phenyl-2-propanol in the 11.5-21.0 GHz frequency range. All three dipole selection rules were observed including 25 a-, 20 b-, and 14 c-type rotational transitions. No unassigned transitions that could be assigned to a second conformation or separate A- and E-torsional states were observed in the spectrum. Nine gauche conformers of (S)-1-phenyl-2-propanol were optimized at the MP2/6-31G* level. These conformations are described by approximate values for the torsional angles τ1) Cφ-C1-C2-O and τ2 ) C1-C2-O-H (g, +60°; g′, -60°; a, 180°). These two torsion angles determine the positions of the hydroxyl and methyl groups as well as the orientation of the hydroxyl hydrogen. The relative energies of the MP2/6-31G* optimized geometries and the corresponding MP2/6-311++G** single-point results [1].
Chemical synthesis of 1-PHENYL-2-PROPANOL
Weigh a mixture of (cyclopentadienone)iron complex (0.05 equivalent, 9.6 mg, 0.025 mmol; kept in the fridge under air) and Me3NO (0.075 equivalent, 2.82 mg, 0.038 mmol; kept in a glove box) into the glass reaction vial. Flush H2 in the vial with the pressure of 50 bar. Keep the temperature of 150 ℃ for 22 h. Add magnetic stir bars in each of them. The yield of 1-PHENYL-2-PROPANOL was 91% [2]. Determined by proton nuclear magnetic resonance (1H NMR) using benzyl benzoate as an internal standard.
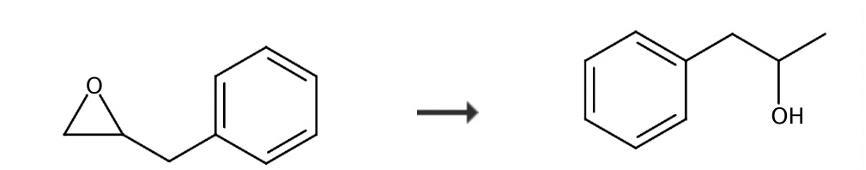
Figure 2 Chemical synthesis of 1-PHENYL-2-PROPANOL.
Biological synthesis of 1-PHENYL-2-PROPANOL
Rhodococcus erythropolis JX-021was grown on an LB agar plate at room temperature for 4 days and inoculated into 120 mL medium containing (g/L) KH2PO4 2.44, Na2HPO4·12H2O 12.03, MgCl2·6H2O 0.412, CaCl2·2H2O 0.001, FeCl3·6H2O 0.001, MnCl2 4H2O 0.004, glycerol 1.12, NH4Cl 2.11, 2% glucose, and 5 mM benzene acetone. The cells were grown at 25 °C and 200 rpm for 36 h, reaching an OD450 of 7.5 in the late exponential phase. The cells of R. erythropolis JX-021 was grown to a cell density of 15.3 g cdw/L according to the procedure described above. 1-PHENYL-2-PROPANOL (1.34 g, 10 mmol, 10 mM) was added to the culture, and the cells grew slowly. It stopped growing after 2 h. 1-PHENYL-2-PROPANOL (1.34 g, 10 mmol, 10 mM) was added every 1 h with a cumulative concentration of 50 mM (6.7 g, 50 mmol, 50 mM) at 5 h. Ten grams of glucose (1%) was added each time, and 60 g of glucose (0.33 mol, 0.33 M) was added after 5 h, and the biotransformation was stopped at 20 h. In analyzing conversion by GC, 300 μL solutions were taken at different time points (0.5, 1, 2, 3, 5, 16, 18, 20 h). The analytic samples were prepared by centrifugation, mixing of 100 μL supernatant with 400 μL Tris buffer (pH=7.0), extracting with 500 μL chloroform, and drying the organic solution with Na2SO4. The frozen cells of R. erythropolis JX-021 were thawed and suspended to a density of 6.7 g cdw/L in 10 mL 100 mM Tris buffer (pH=7.0) containing 2% glucose (200 mg, 1.11 mmol, 111 mM) at pH of 6, 7, and 8, respectively. 1-PHENYL-2-PROPANOL (6.7 mg, 5 mmol, 5 mM) was added, and the mixture was shaken at 25 °C, 300 rpm for 30 min. Cells were removed by centrifugation, 1.0 mL supernatant was extracted with 1.0 mL chloroform, and the organic solvent was dried and analyzed by GC [3].
Analytical method of 1-PHENYL-2-PROPANOL
1-PHENYL-2-PROPANOL was analyzed by gas chromatography (GC; Fisons Instruments) on a fused silica capillary column OPTIMA-5 (25 m × 0.32 mm) with hydrogen as the carrier gas. Temperature program was as follows: 100 °C for 0 min, increase to 280 °C at a rate of 20 °C/min, kept at 280 °C for 2 min. Retention time was 6.22 min for 1-PHENYL-2-PROPANOL. The e.e. of 1-PHENYL-2-PROPANOLwas analyzed by GC on a chiral column CP-Chirasil-Dex CB (25 m × 0.25 mm). Temperature program was as follows: 100 °C to 120 °C at 10 °C/min, increase the temperature to 180 °C at 40 °C/min; retention time was 4.9 min for (S)-1-PHENYL-2-PROPANOL and 6.1 min for (R)-1-PHENYL-2-PROPANOL [3].
References
[1]Tubergen, MJ; Lavrich, RJ et al. Rotational spectra and conformational structures of 1-phenyl-2-propanol, methamphetamine, and 1-phenyl-2-propanone. J. Phys. Chem. A 2006, 110, 13188-13194.
[2]Tadiello, L; Gandini, T et al. Regiodivergent Reductive Opening of Epoxides by Catalytic Hydrogenation Promoted by a (Cyclopentadienone)iron Complex. ACS Catalysis, 2022, 12 (1): 235-246.
[3]Jin, JZ; Li, H and Zhang, J. Improved Synthesis of (S)-1-Phenyl-2-Propanol in High Concentration with Coupled Whole Cells of Rhodococcus erythropolis and Bacillus subtilis on Preparative Scale. Appl Biochem Biotechnol (2010) 162:2075–2086.
Related articles And Qustion
See also

US $0.00-0.00/kg2025-09-10
- CAS:
- 14898-87-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20T

US $80.00/KG2023-08-16
- CAS:
- 14898-87-4
- Min. Order:
- 1KG
- Purity:
- >99%
- Supply Ability:
- 20tons