Niraparib: Uses, Mechanism of action, Efficacy and side effects
What is Niraparib?
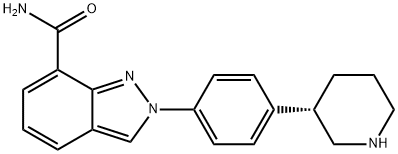
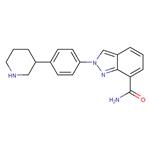
Niraparib is an oral, highly selective PARP1 and PARP2 inhibitor. On April 29, 2020, the Food and Drug Administration approved niraparib (ZEJULA, GlaxoSmithKline) for the maintenance treatment of adult patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to first-line platinum-based chemotherapy. Niraparib has shown efficacy both in patients who have tumors with BRCA mutations and in those without BRCA mutations.
Mechanism of action
Niraparib inhibits the role of PARP enzymes (PARP-1 and PARP-2) in DNA repair. By blocking PARPase activity and increasing PARP-DNA complex formation, niraparib induces DNA damage and cell death. Niraparib-induced cytotoxicity has been demonstrated in tumour cell lines with and without BRCA1/2 gene defects
Efficacy and side effects
PRIMA (NCT02655016) was a double-blind, placebo-controlled trial that investigated the efficacy of 733 patients. All patients were complete or partial responders to first-line platinum-based chemotherapy.
The primary efficacy metric, progression-free survival (PFS), was tested first in the homologous recombination-deficient population and then in the overall population, and was determined by blinded independent central review according to RECIST 1.1. Tumour samples were tested for homologous recombination defect status; a tumour was considered homologous recombination defective if it had a mutation in the breast cancer susceptibility gene (tBRCA) or a genomic instability score (GIS) ≥42. Treatment of this indication with ZEJULA does not require an FDA-approved companion diagnosis.
The trial demonstrated a statistically significant improvement in PFS for patients randomised to niraparib compared to placebo in both the homologous recombination-deficient population and the overall population. In the homologous recombination-deficient population, patients treated with niraparib had a median survival of 21.9 months (19.3 months, NE), compared with 10.4 months (8.1 months, 12.1 months) for patients treated with placebo (HR 0.43; 95% CI: 0.31, 0.59; P<0.0001). Patients treated with niraparib had a median overall survival of 13.8 months (11.5-14.9 months), compared with a median overall survival of 8.2 months (7.3-8.5 months) for patients treated with placebo (HR 0.62; 95% CI: 0.50-0.76; p<0.0001).
In the PRIMA trial, in all patients treated with niraparib, the most common adverse reactions were thrombocytopenia, anaemia, nausea, malaise, neutropenia, constipation, musculoskeletal pain, leucopenia, headache, insomnia, vomiting, dyspnoea, loss of appetite, dizziness, cough, hypertension, elevated glutamine/glutamine aminotransferase and acute kidney injury.
In a pooled safety population of patients (n = 1,314) with advanced ovarian, fallopian tube, or
primary peritoneal cancer treated with ZEJULA monotherapy including PRIMA (n = 484),
NOVA (n = 367), and another clinical trial (n = 463) , the most common adverse reactions >10%
were nausea (65%), thrombocytopenia (60%), anemia (56%), fatigue (55%), constipation (39%),
musculoskeletal pain (36%), abdominal pain (35%), vomiting (33%), neutropenia (31%),
decreased appetite (24%), leukopenia (24%), insomnia (23%), headache (23%), dyspnea (22%),
rash (21%), diarrhea (18%), hypertension (17%), cough (16%), dizziness (14%), acute kidney
injury (13%), urinary tract infection (12%), and hypomagnesemia (11%).
The recommended dose of niraparib for first-line maintenance therapy in advanced ovarian cancer is based on body weight or platelet count. For patients weighing less than 77 kg (170 lbs) or with a platelet count less than 150,000/μL, the recommended dose is 200 mg orally once daily. For patients weighing 77 kg (170 lbs) or more and with a platelet count greater than or equal to 150,000/μL, the recommended dose is 300 mg orally once daily.
References:
[1] SHIMADA M, KUWAHARA Y, KUWABARA H, et al. MO71-4 Real-world treatment of niraparib as maintenance therapy in patients newly diagnosed advanced ovarian cancer in Japan[J]. Annals of Oncology, 2023. DOI:10.1016/j.annonc.2023.09.327.
[2] A. G. MARTÍN. Niraparib therapy in patients with newly diagnosed advanced ovarian cancer (PRIMA/ENGOT-OV26/GOG-3012 study)[J]. Annals of Oncology, 2019. DOI:10.1093/annonc/mdz394.052.
You may like
See also
Lastest Price from Niraparib manufacturers
US $0.00-0.00/g2025-04-20
- CAS:
- 1038915-60-4
- Min. Order:
- 10000g
- Purity:
- 99% HPLC
- Supply Ability:
- 1000kg

US $0.00-0.00/KG2025-04-15
- CAS:
- 1038915-60-4
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg