What is glyoxylic acid used for hair?
Introduction
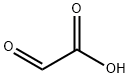
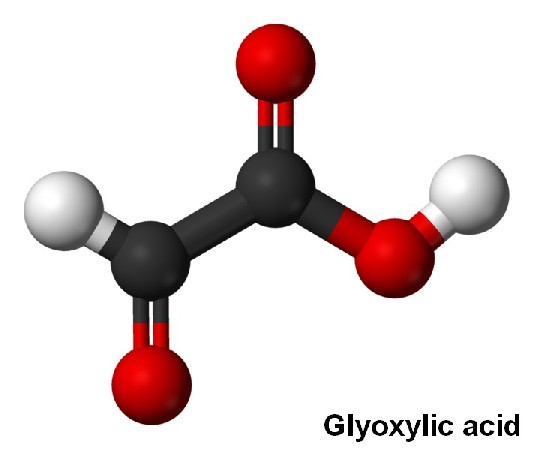
Glyoxylic acid is an essential intermediate of organic chemicals, and it can generate dozens of fine chemical products. It is a tartaric acid degradation product formed in model wine solutions containing iron, and its production is significantly increased by exposure to UV–visible light. Glyoxylic acid and its derivatives are commonly used as decarboxylative coupling reagents to synthesize aldehydes or ketones. Glyoxylic acid and its acetal derivatives are low toxicity, easy to handle and store, highly stable, and readily available [1].

Deterioration of white wine
The photochemical reaction that may contribute to the light-induced deterioration of white wine is the photodegradation of iron(III) tartrate. In model wine solutions, this reaction produces glyoxylic acid, a two-carbon carboxylic acid with an aldehyde group. Glyoxylic acid can react with nucleophiles in wine, such as flavan-3-ols ((+)- catechin and (–)-epicatechin), phenolic compounds originating from grape skins and seeds, and also hydrogen sulfite, the primary form of sulfur dioxide in wine pH[2]. The reaction of glyoxylic acid with flavan-3-ols ultimately generates yellow-colored xanthylium cations. Chardonnay wine supplemented with (+)-catechin exposed to light in clear glass bottles was reported to have higher levels of these pigments than samples in green glass bottles, which transmit less light. At wine pH, glyoxylic acid reacts reversibly with hydrogen sulfite to form a relatively stable addition product, also known as an α-hydroxy sulfonate, resulting, in wine, in a decrease in the amount of free sulfur dioxide.
For hair
Glyoxylic acid (GA) is widely used as a straight perming agent for hair care products; it penetrates deeply to repair, nourish, and reshape the hair shaft, allowing an intense and long-lasting smoothing action. Its molecules are activated by heat and create a protective net around the hair, keeping it smooth for up to 3 months from the date of application of treatment. However, advanced GA penetration-enhancing agents are desired due to the peculiar odor and hair color fading caused by the continuous use of GA products. Hence, developing a penetration-enhancing agent that helps minimize the GA concentration is essential.
Straightening hair tests indicated that the hair straightening effect by GA was enhanced by the presence of glycolic acid (GCA). LC/MS results showed that adding GCA enhanced the amount of GA that penetrated human hair by about four times[3]. ATR FT-IR and FT-IR microscope measurements indicated that GA was localized more in the innermost hair region (medulla) than the cortex and cuticle. Due to the chemical reaction, the GA accumulated in the medulla disappeared after a hair straightener treatment at 180°C. Hence, the GA penetration-enhancing effect of GCA is worth investigating to reduce the GA concentration in products for more comfortable use.
References
[1] Yunyu Xiang. “Decarboxylative coupling of glyoxylic acid and its acetal derivatives: A unique C1 formylation synthon.” Tetrahedron 91 (2021): Article 132193.
[2] Paris Grant-Preece. “Photoproduction of glyoxylic acid in model wine: Impact of sulfur dioxide, caffeic acid, pH and temperature.” Food Chemistry 215 (2017): Pages 292-300.
[3] Makoto Uyama. “Promotion of glyoxylic acid penetration into human hair by glycolic acid.” International Journal of Cosmetic Science 45 2 (2023): 246–254.
You may like
Related articles And Qustion
See also
Lastest Price from Glyoxylic acid manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 298-12-4
- Min. Order:
- 1KG
- Purity:
- 50.30%
- Supply Ability:
- 50tons/month

US $10.00/kg2025-04-21
- CAS:
- 298-12-4
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON