Glyoxylic acid: Application, Production and Isolation
General Description
Glyoxylic acid is a colourless solid that occurs naturally and is useful industrially. Aqueous solution of Glyoxylic acid is transparent colorless or light yellow liquid. Soluble in water and ethanol, slightly soluble in organic solvents like ether or benzene, insoluble in esters aromatic solvents. This solution is not stable but will not decay in the air. Glyoxylic acid is a strong organic acid and a highly reactive chemical intermediate having two functional groups: the aldehyde group and the carboxylic acid group. Because of its bi-functionality is a versatile reagent in organic and íne chemicals syntheses.1

Figure 1. Properties of Glyoxylic Acid Monohydrate
Applications
Electroless Copper Plating
Glyoxylic acid can be used as an alternative reducing agent for electroless copper plating. Plating rates and bath stability were superior to that of the formaldehyde bath under standard conditions. Glyoxylate ions in the plating bath have no vapor pressure and show good reducing power in the electroless copper plating. Therefore, glyoxylic acid can replace formaldehyde, and eliminate health and environmental problems resulting from generation of the fumes.2
Biogenic Amines Visualizer
Glyoxylic acid can undergo various condensation reactions and thus is commonly used for visual detection of biogenic amines in histological sections. This is the sucrose-phosphate-glyoxylic acid (SPG) histoîuorescence method for the visualization of monoamines in tissues where the fluorescence is analyzed by fluorescence microscopy. It is used for counterstaining tissues.3
Fungicides
Glyoxylic acid derivatives have been described as a new class of Oomycete fungicides. The GXA derivatives exhibit speciíc activity against oomycetes, including dwony mildew on grapes (Plasmopara viticola) and late blight on potatoes an tomatoes (Phytophthora infestans). They are active against Oomycetes in soil such as Phytophthora in tabacoo and citrus. They exhibit protective, curative, eradicative and antisporulant activity. This class of compounds can be synthesized via a simple synthetic approach where in the second step the glycolic acid ester is needed.4
Pharmaceuticals
Gloxylic acid is either a raw material or used as an intermediate in the synthesis of various pharmaceutical products like D,L-phydroxyphenylglycine, D,L-p-hydroxyphenylhydantoin, orotic aid, sulindac, mandelic acid, p-hydroxyphenylacetic acid, diphenylacetic acid, Lamivudine and 4-Aminophenylacetic acid.5
Agrochemicals
Glyoxylic acid is used as a key raw material for the synthesis of e.g. Ethylene bis(hydroxyphenyl)glycine, glyphosate, 2-hydroxy quinoxaline and complexing agents like EDDHA.6
Flavor and Fragrance Products
Glyoxylic acid can be used as a key raw material for diìerent aromas such as vanillin, ethyl vanillin and heliotropin.7
Personal Care Products Ingredients
A very prominent product produced from glyoxylic acid is Allantoin which is used to a great extent in cosmetics. It can be considered as the diureide of glyoxylic acid and is highly active in skin-softening (keratolytic effect) and rapid cell regeneration by precipitating proteins on skin. Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids.8
Biological role
Glyoxylic acid is an intermediate of the glyoxylate cycle, which enables certain organisms to convert fatty acids into carbohydrates. The conjugate base of glyoxylic acid is known as glyoxylate. In humans, glyoxylate is produced via two pathways: (1) through the oxidation of glycolate in peroxisomes and (2) through the catabolism of hydroxyproline in mitochondria. In the peroxisomes, glyoxylate is converted into glycine by glyoxylate aminotransferase (AGT1) or into oxalate by glycolate oxidase. In the mitochondria, glyoxylate is converted into glycine by mitochondrial glyoxylate aminotransferase AGT2 or into glycolate by glycolate reductase. A small amount of glyoxylate is converted into oxalate by cytoplasmic lactate dehydrogenase. Glyoxylic acid is found to be associated with primary hyperoxaluria I, which is an inborn error of metabolism. Under certain circumstances, glyoxylate can be a nephrotoxin and a metabotoxin. A nephrotoxin is a compound that causes damage to the kidney and kidney tissues. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. High levels of glyoxylate are involved in the development of hyperoxaluria, a key cause of nephrolithiasis (commonly known as kidney stones). Glyoxylate is both a substrate and inductor of sulfate anion transporter-1 (SAT-1), a gene responsible for oxalate transportation, allowing it to increase SAT-1 mRNA expression, and as a result oxalate efflux from the cell. The increased oxalate release allows the buildup of calcium oxalate in the urine, and thus the eventual formation of kidney stones. As an aldehyde, glyoxylate is also highly reactive and will modify proteins to form advanced glycation products (AGEs).9
Synthesis and Isolation
Initially, oxalic acid was the most widely used starting compound for glyoxylic acid synthesis. Experimental results of several research teams indicate that glyoxylic acid synthesis by the electroreduction of oxalic acid in aqueous solutions is possible on cathodes manufactured from Ti and Pt, graphite, Pb, Pb/Tl alloy, TiO2, and Pb with addition of Pb2+ salts to the solution. Apart from the electrode material, the process is considerably aff ected by the temperature and cathode voltage. As noted above, the cathodic reduction of oxalic acid and anodic oxidation of glyoxal can be accomplished simultaneously in the same reactor. The necessity to vary a large number of parameters complicates selection of the optimal conditions of synthesis, which also results in rather low yields. One more drawback of the method is fast cathode deactivation, which is associated with additional cost.10
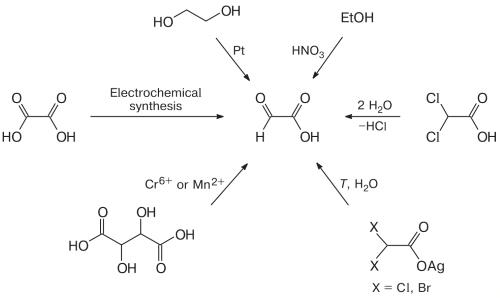
Figure 2. Synthetic Routes of Glyoxylic Acid
The isolation of glyoxylic acid from the products of synthesis is a complicated engineering process based on various methods. The isolation of glyoxylic acid is a challenge, as the aqueous solution contains simultaneously the initial and auxiliary reagents, and target and side reaction products, which aqueous solutions of glyoxal oxidation products are treated with a precipitating agent (Ca2+ source) to generate a mixture of poorly soluble precipitates of calcium glyoxylate and oxalate; after that, the resulting mixture is added to an oxalic acid solution at 20—80oC. As a result of exchange reaction, oxalic acid is converted to insoluble calcium oxalate, which is separated by fi ltration; glyoxylic acid remaining in the fi ltrate is concentrated. The separation method is applicable to any syntheses that give oxalic acid together with glyoxylic acid; it does not require specifi c equipment or ion exchange resins and can be used for solutions containing glyoxylic acid in a broad concentration range.11
Crystallization
The glyoxylic acid crystallization is attained using drying agents; this gives rise to irregular-shaped orthorhombic crystals of glyoxylic acid monohydrate. It is also known that storage of a concentrated solution of glyoxylic acid over fresh P2O5 results in complete removal of the solvent and formation of glyoxylic acid crystals within several months. Preparation of glyoxylic acid as the sodium-calcium salt is also possible. Recrystallization from hot water and treatment with 2,4-dinitrophenylhydrazine aff ords glyoxylic acid crystals (79%). This is a multistep synthesis requiring a long time. The monohydrate was prepared by a procedure involving evaporation of water and formic acid (formed as a by-product) after glyoxylic acid synthesis at a reduced pressure at 50 oC and the subsequent addition of seed crystals with stirring at 20 oC; this gave glyoxylic acid monohydrate in a yield of up to 97%. For the preparation of crystals of glyoxylic acid monohydrate, commercial crystalline glyoxylic acid and heavy water are added to a solution of freshly prepared glyoxylic acid, the mixture is kept in the reactor for a long time at a temperature from 20 to 80 oC; after that, water is evaporated at a reduced pressure. The above methods for the preparation of crystalline glyoxylic acid monohydrate require a long time and sophisticated equipment.12
References
1. S. V. Sidorenko, S. V. Yakovlev, Rus. Med. Zh. [Russ. Med. J.], 1997, 21, 2 (in Russian).
2. G. Mattioda, Y. Christidis, Glyoxylic Acid. Ullmann´s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000, 89-92.
3. C. Escher, F. Widmer, J. Biol. Chem., 1997, 378, 803.
4. P. Gallezot, R. de Mesanstourne, Y. Christid, G. Mattioda, A. Schouteeten, J. Catal., 1992, 133, 479.
5. P. Gallezot, F. Fache, R. de Mesanstourne, Y. Christidis, G. Mattioda, A. Schouteeten, Stud. Surf. Sci. Catal., 1993, 75, 195.
6. Z. Li, D. Huang, X. Ruimin, W. Liu, C. Xu, Y. Jiang, L. Sun, Curr. Nanosci., 2012, 8, 26.
7. Z. Li, D. Huang, W. Liu, Y. Min, R. Xiao, Y. Jiang, L. Sun, Mater. Sci. Forum, 2011, 694, 288.
8. M. L. Jia, C. X. Liu, J. Wang, S. Bao, Z. Bao, Kinet. Catal., 2014, 55, 671.
9. Schnedler, Nina; Burckhardt, Gerhard; Burckhardt, Birgitta C. Journal of Hepatology. 2011, 54 (3): 513–520.
10. A. J. Hoefnagel, PhD Thesis, Delft University of Technology, Delft, The Netherlands, 1993, 192.
11. M. A. Pozdniakov, K. V. Rubtsov, V. V. Botvin, A. A. Sorvanov, A. S. Knyazev, A. G. Filimoshkin, Sep. Sci. Technol., 2016, 52, 876.
12. W. Black, G. Cook, Ind. Eng. Chem. Dev., 1966, 5, 350.
Related articles And Qustion
See also
Lastest Price from Glyoxylic acid manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 298-12-4
- Min. Order:
- 1KG
- Purity:
- 50.30%
- Supply Ability:
- 50tons/month

US $10.00/kg2025-04-21
- CAS:
- 298-12-4
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 100 TON