Nintedanib:Class, Uses, Synthesis, Mechanism of Action and Toxicity
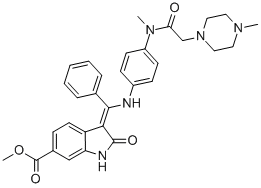
Nintedanib is a small molecule tyrosine kinase inhibitor (TKI) that binds to a family of growth factor receptors and prevents the proliferation of fibroblasts. It impedes the ongoing fibrotic process and delays the progression to long-lasting damage.

Class
Multikinase inhibitors
Uses
Nintedanib was initially approved by the U.S. Food and Drug Administration (FDA) in 2014 as a treatment for idiopathic pulmonary fibrosis (IPF). Subsequently, nintedanib was noted to have antifibrotic effects in animal models of rheumatoid arthritis-associated interstitial lung disease (RA-ILD) and systemic sclerosis-associated interstitial lung disease (SSc-ILD). Interest in this antifibrotic property led to clinical trials demonstrating significant slowing of fibrosis in ILD related to systemic autoimmune rheumatic diseases (SARD), including systemic sclerosis. Study results also indicated that nintedanib therapy slows the decline of forced vital capacity (FVC).[1]
Structure and Synthesis
The convergent syntheses of nintedanib:
The reaction scheme shown in Figure 2 depicts an optimized synthesis route that can be used for the preparation of >100 g batches of the compounds.
After a classical malonic ester addition to arene 3, the resulting nitro benzene (4) is hydrogenated under acidic conditions, furnishing the 6-methoxycarbonyl-substituted oxindole 5 via decarboxylative cyclization. Condensation of 5 with trimethyl orthobenzoate in acetic anhydride leads to compound 6, one of the two key building blocks of the synthesis. The concomitant N-acetylation of the oxindole activates the scaffold for the condensation reaction.
The aniline side chain (9) can be prepared by a one-pot bromo-acetylation/amination of the para-nitro-phenylamine (7) using bromoacetyl bromide and N-methylpiperazine and a subsequent hydrogenation furnishing 9 as the second key building block. Condensation of both building blocks in an addition–elimination sequence and subsequent acetyl removal with piperidine furnishes 2 as free base (pKa = 7.9), which subsequently is converted into its monoethanesulfonate salt (1). Compound 1 is highly crystalline (mp = 305 °C) and exhibits a log P of 3.0 and good aqueous solubility (>20 mg/mL in water).

Figure 2 Synthesis of nintedanib ethanesulfonate
Mechanism of Action
Nintedanib is an indolinone derivative that binds to tyrosine kinase receptors. The mechanism of action is thought to involve the restriction of neoangiogenesis by inhibiting various growth factors such as fibroblast growth factor receptor (FGFR), vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), colony-stimulating factor-1-receptor (CSF1R), and Fms-like tyrosine kinase-3 (FLT3).Nintedanib competitively binds to the intracellular adenosine triphosphate (ATP) binding site of the growth factor receptors, preventing autophosphorylation and blocking downstream signaling cascades. Additionally, nintedanib blocks non-receptor tyrosine kinases (eg, Src, Lck) directly, preventing the activation of fibroblasts.
Toxicity
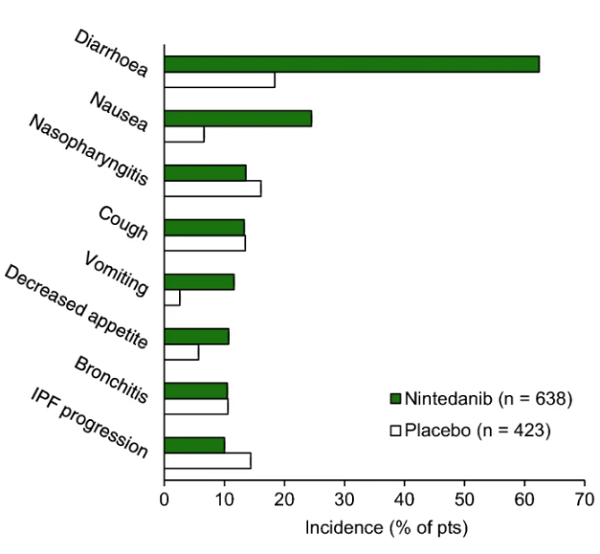
Nintedanib therapy is frequently associated with elevated liver enzymes and bilirubin, but this is usually reversible with dose reduction or treatment interruption. For patients with ALT/AST elevations of less than 3 to 5 times the upper limit without signs of hepatotoxicity, therapy should be held and resumed at a lower dose of 100 mg twice daily once the liver enzymes normalize. Women with low BMI, low body surface area (BSA), or Asian ethnicity are at higher risk of hepatotoxicity when treated with 150 mg twice daily. This is reversible with treatment interruption or dose reduction. If there is no hepatotoxicity, these patients may also benefit from starting at 100 mg twice daily with dose titration.[2]
References
[1] Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N Engl J Med. 2015 Aug 20;373(8):782.
[2]Ikeda S, Sekine A, Baba T, Yamanaka Y, Sadoyama S, Yamakawa H, Oda T, Okuda R, Kitamura H, Okudela K, Iwasawa T, Ohashi K, Takemura T, Ogura T. Low body surface area predicts hepatotoxicity of nintedanib in patients with idiopathic pulmonary fibrosis. Sci Rep. 2017 Sep 07;7(1):10811.
You may like
Related articles And Qustion
Lastest Price from BIBF-1120 manufacturers

US $0.00/kg2025-04-02
- CAS:
- 928326-83-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $15.00-10.00/KG2021-07-13
- CAS:
- 928326-83-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton