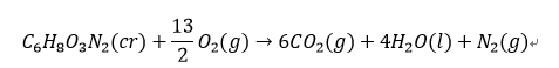Electrochemical Reactivity of 1,3-Dimethylbarbituric Acid as a Carbon-Centered Nucleophile
Structure−Energy Relationship in 1,3-Dimethylbarbituric Acid
Barbituric acid derivatives possess a rather broad spectrum of therapeutic activity. They are used as sedatives, hypnotics, soporifics, anticonvulsants, or as adjuncts in anesthesia. The behavior of the 1,3-Dimethylbarbituric acid as a function of temperature was studied by differential scanning calorimetry. DSC was used to study the possible existence of phase transitions in the solid sample of 1,3-dimethylbarbituric acid. No phase transitions were observed in the temperature interval between T = 268 K to T = 396 K, the melting temperature. The fusion temperature(Tfus) is 396.1 ± 0.3 K, the enthalpy(ΔfusH(Tfus)) is 17.7 ± 0.1 kJ·mol−1 , and the experimental entropy of fusion(ΔfusS(Tfus)expt,) is 44.6 ± 0.3 J·K−1·mol−1. Results for the combustion experiments on 1,3-dimethylbarbituric acid are correspond to the reaction:
The table lists the derived standard molar enthalpies of combustion and formation in the crystalline state at T = 298.15 K. [1]
Molecular and Electronic Structure
1,3-Dimethylbarbituric acid contains only one enolizable hydrogen atom, and so it may exist in two tautomeric forms differing by the position of the hydrogen, which may be bound to a carbon or an oxygen atom. The higher stability of the triketo form is associated with the much stronger double bond of C═O compared with the strength of the C═C bond. This high energy difference suggests that the gas phase of 1,3-dimethylbarbituric acid consists of a single molecular species. The crystal structure of 1,3-dimethylbarbituric acid was reported by Bertolasi et al. The structure is orthorhombic of the space group Fdd2, a = 15.642, b = 29.006, c = 6.5560 Å, with Z = 16. 1,3-Dimethylbarbituric acid does not enolize in crystals. In contrast to barbituric acid, the crystal structure of 1,3-dimethylbarbituric acid does not contain any traditional H-donor group that would enable formation of strong hydrogen bonding interactions. It forms crystals whose packing is dominated by perpendicular donor−acceptor Cδ+═Oδ−···Cδ+═Oδ− interactions and C—H···O═C hydrogen bonds. [1]
Solid–gas Reactions between 1,3-dimethylbarbituric Acid and Amines
The gas–solid reactions between 1,3-dimethylbarbituric acid (DMBA) and the volatile amines yield the polycrystalline dimethylbarbiturate (DMB) salts: [NH4]DMB (1), [NH3(CH3)]DMB (2) and [NH2(CH3)2]DMB (3), which have been investigated by powder diffraction, DSC, TGA as well as UV–Vis spectroscopy. Well shaped crystals of [NH4]DMB, suitable for single-crystal X-ray diffraction, were obtained by recrystallization in methanol of the powder obtained via the heterogeneous reaction of ,3-dimethylbarbituric acid with NH3. The dimethylbarbiturate anion is disordered over three orientations, thus generating a crystallographic ternary axis. As a matter of fact, the structure has been solved in the trigonal crystal system P3c1. The dimethylbarbiturate anions are organized in layers, which are then stacked on top of each other at the interplanar distance of 3.58 Å. [2]
![Article illustration]()
![Article illustration]() 1,3-dimethylbarbituric acid as Nucleophiles
1,3-dimethylbarbituric acid as Nucleophiles
Because electrochemical oxidation very often parallels the cytochrome P450 catalyzed oxidation in liver microsomes, it was interesting to study the anodic oxidation of catechol in the presence of the barbiturates that could serve as carbon-centered pro-nucleophiles. In recent work electrochemical oxidation of 4-tert-butylcatechol has been studied in the presence of 1,3-dimethylbarbituric acid and 1,3-diethyl-2-thiobarbituric acid as possible nucleophiles. The results of this work show that 4-tert-buthylcatechol is oxidized in water to its o-quinone. The quinone is then attacked by the enolate anion of 1,3-dimethylbarbituric acid or 1,3-diethyl-2-thiobarbituric acid to form spiropyrimidine derivatives. The overall reaction mechanism for anodic oxidation of 4-tert-buthylcatecho in the presence of barbituric acids as the nucleophile. The Michael addition reaction of these nucleophiles to the o-quinone formed leads to the formation of new and unique spiropyrimidine derivatives as final products, in good yield and purity. [3]
References:
[1] Roux, M. V., Notario, R., Foces-Foces, C., Temprado, M., Ros, F., Emel'Yanenko, V. N., & Verevkin, S. P. (2011). Experimental and Computational Thermochemical Study of Barbituric Acids: Structure? Energy Relationship in 1, 3-Dimethylbarbituric Acid. The Journal of Physical Chemistry A, 115(14), 3167-3173.
[2] Braga, D., Cadoni, M., Grepioni, F., Maini, L., & van de Streek, J. (2007). Solid–gas reactions between 1, 3-dimethylbarbituric acid and amines. A structural and spectroscopic study. New Journal of Chemistry, 31(11), 1935-1940.
[3] Nematollahi, D., & Goodarzi, H. (2001). Electrochemical study of 4-tert-butylcatechol in the presence of 1, 3-dimethylbarbituric acid and 1, 3-diethyl-2-thiobarbituric acid. Application to the electro-organic synthesis of new corresponding spiropyrimidine derivatives. Journal of Electroanalytical Chemistry, 517(1-2), 121-125.
You may like
See also
Lastest Price from 1,3-Dimethylbarbituric acid manufacturers

US $0.00-0.00/Kg2025-04-21
- CAS:
- 769-42-6
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $22.00/kg2025-04-21
- CAS:
- 769-42-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100mt