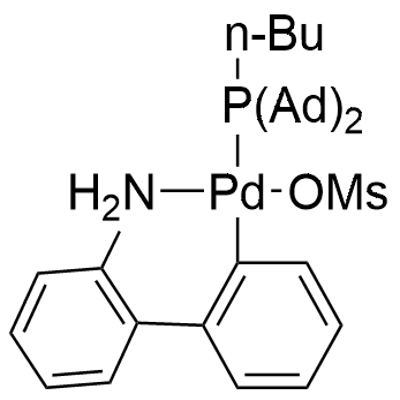
CataCXium A Pd G3: Synthesis and Catalytic Applications
CataCXium A Pd G3, a palladium metal complex, is a beige solid powder under ambient conditions and exhibits unique catalytic properties, commonly employed as a transition metal catalyst for coupling reactions and functionalization of inert chemical bonds. As a versatile precursor, cataCXium A Pd G3 can also be used to prepare other types of palladium-based catalysts.
Synthesis
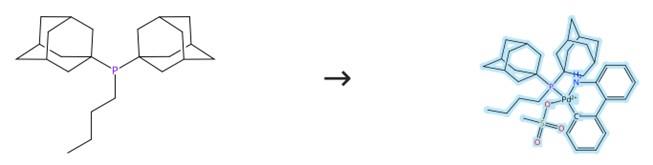
Figure1: Picture of CataCXium A Pd G3
To a 10 mL microwave vial were added μ-mesylate aminobiphenyl palladium dimer (277 mg) and CataCXium® A (269 mg). The vial was sealed, evacuated and back-filled with argon three times, then charged with degassed acetone (3 mL) via freeze-pump-thaw cycling. The reaction mixture was stirred overnight at room temperature, after which the resulting precipitate was collected by vacuum filtration and sequentially washed with acetone and pentane to give product CataCXium A Pd G3. [1]
Catalytic Applications
Sonogashira Reactions
A robust and general protocol was reported for sustainable copper-free Sonogashira cross-coupling in micellar aqueous media, achieving high catalytic turnover. This method utilizes the commercially available CataCXium A Pd G3 as the palladium source with THF as cosolvent, enabling efficient coupling of diverse alkynes with aryl halides across a broad substrate scope while maintaining high yields and low catalyst loadings. Through systematic parameter optimization, the process demonstrates operational simplicity, excellent robustness, and scalability. The application of CataCXium A Pd G3 in micellar aqueous conditions proved crucial for achieving superior selectivity in synthesizing alkynylated arenes, heterocycles, and monofunctionalized products from dihalogenated precursors. [2]
Experimental Procedure
A solution of 2-bromoiodobenzene (5.00 g, 17.7 mmol) in 2 wt% aqueous TPGS-750-M (75.0 mL) was charged with CataCXium A Pd G3 (25.7 mg, 35.4 μmol, 0.20 mol%), glucose (159 mg, 884 μmol, 5 mol%), THF (11.2 mL), and 3-butyn-1-ol (1.49 g, 21.2 mmol). The resulting mixture was stirred at room temperature for 5 minutes, followed by the addition of triethylamine (7.35 mL, 53.0 mmol). After stirring at 45 °C for 22 hours, the reaction mixture was extracted with ethyl acetate (2 × 50 mL). The combined organic extracts were washed with brine (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. Purification of the residue by column chromatography on silica gel (eluent: methyl tert-butyl ether/heptane, 5:1) afforded the product as a yellowish oil (3.52 g, 88% yield). [2]
Suzuki-Miyaura polycondensation
CataCXium A Pd G3 catalyst was more effective than t-Bu3PPd catalyst for unstoichiometric Suzuki-Miyaura coupling polymerization of excess dibromofluorene with pinacol fluo renediboronate or pinacol benzothiadiazoleboronate, affording high-molecular-weight conjugated polymers with pinacol boro nate ends. Furthermore, polymerization of excess dibromo cyclopentadithiophene and pinacol benzothiadiazolediboronate with CataCXium A Pd G3 yielded high-molecular-weight donor acceptor conjugated alternating copolymer with boronate ends. The unstoichiometric Suzuki-Miyaura polycondensation between dibromo monomers and diboronic acid esters proceeds via alternating intramolecular and intermolecular catalyst transfer processes. Consequently, employing a palladium catalyst exhibiting moderately reduced interaction with the π-face of the polymer backbone promotes the intermolecular catalyst transfer step, leading to higher molecular weight polymers bearing boronic acid ester termini. However, severely diminished interaction between the palladium catalyst and the aromatic π-system would impede even the intramolecular catalyst transfer on dibromo monomers. Previous studies on the Suzuki-Miyaura coupling of 2,5-dibromothiophene with phenylboronic acid demonstrated that CataCXium A Pd G3 afforded a slightly lower diphenylthiophene/monophenylthiophene product ratio compared to Pd/P(t-Bu)₃. This result implied that the former Pd catalyst has slightly lower interaction with aromatic π-face than does CataCXium A Pd G3. Researchers have investigated Suzuki-Miyaura unstoichometric p olycondensation with CataCXium A Pd G3 and found that this catalyst was more effective than PdP(t-Bu)3 for obtaining high-molecular-weight poly f luorene, poly(fluorene-alt-benzothiadiazole), and also poly(cy clopentadithiophene-alt-benzothiadiazole) with a boronic acid ester at each end. [3]
Reference
[1] Johnston, Adam J. S.; et al, Direct ortho-Arylation of Pyridinecarboxylic Acids: Overcoming the Deactivating Effect of sp2-Nitrogen, Organic Letters 2016, 18, 6094-6097.
[2] Parmentier M, et al. A General Protocol for Robust Sonogashira Reactions in Micellar Medium[J]. Helvetica chimica acta, 2019, 102, e1900024.
[3] Kosaka K, et al. Importance of the balance of interaction between palladium catalyst and aromatic π-face for unstoichiometric Suzuki-Miyaura coupling polymerization: effective Pd cataCXium A catalyst for fluorene and cyclopentadithiophene monomers[J]. Chemistry Letters, 2018, 47: 1040-1043.
You may like
See also
US $2.00-5.00/kg2025-06-20
- CAS:
- 1651823-59-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00/G2025-04-21
- CAS:
- 1651823-59-4
- Min. Order:
- 10G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month