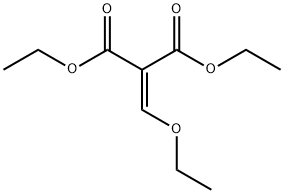
Synthesis and Application of Diethyl ethoxymethylenemalonate
Diethyl ethoxymethylenemalonate, a transparent colorless to pale yellow liquid under ambient conditions, serves as an important organic building block containing multiple reactive functional groups. Owing to its versatile reactivity, diethyl ethoxymethylenemalonate readily undergoes polycondensation and cyclocondensation with diverse compounds, making it particularly valuable for the synthesis of pharmaceutical molecules and bioactive agents such as norfloxacin and lomefloxacin.
Introduction
Diethyl ethoxymethylenemalonate is a very versa tile reagent, extensively used for the synthesis of heterocyclic systems. The main application of this reagent is its use in the Gould–Jacobs reaction.
Synthesis
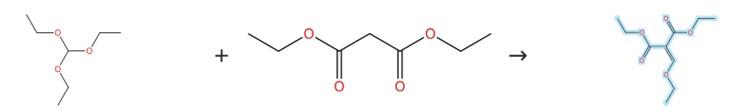
Figure1: Synthesis of Diethyl ethoxymethylenemalonate
Stir a mixture of diethyl malonate (20.0 g, 0.12 mol), triethyl orthoformate (35.6 g, 0.24 mol) and acetic anhydride (24.5 g, 0.24 mol) at 140°C for 5 hours using zinc(II) chloride as catalyst. Monitor the completion of reaction by TLC, petroleum. Evaporate the generated ethanol and acetic acid under reduced pressure to obtain diethyl ethoxymethylenemalonate. [1]
Application
Chiral Resolution for Amino Acids
Diethyl ethoxymethylenemalonate was employed as the derivatizing agent in a precolumn methodology for amino acid determination, combining reversed-phase high-performance liquid chromatography (HPLC) with detection at 290 nm. The derivatives generated using diethyl ethoxymethylenemalonate remained stable at ambient temperature following a 50-minute reaction period. Effective chromatographic separation of derivatives from seventeen amino acids—including proline and cystine—was accomplished within 35 minutes utilizing a binary gradient elution system, achieving a detection limit of 3 pmol. Comparative analysis of acid-hydrolyzed protein samples demonstrated full agreement between this approach and conventional ion-exchange chromatography, while the operational simplicity enables implementation on standard multi-purpose HPLC instrumentation. In conclusion, the derivatization of amino acids with diethyl ethoxymethylenemalonate and their subsequent reversed-phase HPLC is recommended as an alternative to the dedicated amino acid ana- lyser when subpicomole sensitivity is not required. [2]
Chiral Resolution of Aliphatic Diamines
Aliphatic diamines serve as key chemical reagents for polyamide monomers (e.g., Nylon 46, 510, 66, 610) and can be biosynthesized from lysine, arginine, or ornithine via microbial decarboxylase metabolism, yet conventional derivatization methods for HPLC-based diamine quantification lack sufficient sensitivity and efficiency for precise enzyme activity monitoring. In this study, diethyl ethoxymethylenemalonate (DEEMM) was employed as a derivatizing agent to track lysine decarboxylase activity during cadaverine production by quantifying intracellular and secreted lysine and cadaverine levels. Through this highly sensitive approach, diethyl ethoxymethylenemalonate enabled the establishment of linear calibration curves for twelve amine compounds—including 2,4-diaminobutyrate, 2,6-diaminopimelic acid, ornithine, arginine, lysine, 1,3-diaminopropane, putrescine dihydrochloride, cadaverine, hexamethylenediamine, and 1,7-diaminoheptane—achieving detection limits as low as 0.001 mM. This robust methodology provides an effective alternative for sensitive monitoring of lysine decarboxylase kinetics via HPLC-UV through simultaneous detection of amine-containing substrates and diamine products. [3]
Synthesis of polysubstituted-2-pyridones
Diethyl ethoxymethylenemalonate is a useful regent for synthe sis of heterocyclic compounds.7 However, most literature reported that the first step of formation of heterocyclic compounds is ethoxy group substituted by nitrogen. Recently, Poudel reported 4-oxo-4H-chromene-3-carbaldehydes with 1,3-diketoesters and anilines for the synthesis of 2-pyridone derivatives. In this reaction mechanism, firstly, amine was as ambident nucleophiles to open the cycle, then heterocycles were formed through ring closure. A simple and novel synthesis of polytrisubstituted-2-pyridones derivatives by the reaction of enamino esters and diethyl ethoxymethylenemalonate has been reported. The method provides a rapid synthetic route for the construction of 2-pyridones under catalyst- and solvent-free reaction conditions in moderate-to-good yields. Typical experimental procedure: A sealed test tube was charged with ethyl 3-(phenylamino)but-2-enoate (205 mg, 1.0 mmol) and Diethyl ethoxymethylenemalonate (216mg, 1mmol) and the resulting mixture heated at 130 C for 6 h under a nitrogen atmosphere. After the disappearance of reactants (as monitored by TLC), the mixture was directly purified by column chromatography on silica gel (petroleum ether/ EtOAc = 5:1) to yield the desired product 3a (203.2 mg, 62%) as an off-white solid. [4]
Reference
[1] Zang, Zhong-Lin; et al, Synthesis and antibacterial medicinal evaluation of carbothioamido hydrazonyl thiazolylquinolone with multitargeting antimicrobial potential to combat increasingly global resistance, European Journal of Medicinal Chemistry 2024, 275, 116626.
[2] Alaiz M, et al. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate[J]. Journal of Chromatography A, 1992, 591: 181-186.
[3] Yong Hyun Kim, Application of diethyl ethoxymethylenemalonate (DEEMM) derivatization for monitoring of lysine decarboxylase activity, Journal of Molecular Catalysis B: Enzymatic, 2015, 115, 151-154.
[4] Xian-Liang Zhao, A new synthetic approach to polysubstituted-2-pyridones from enamino esters and diethyl ethoxymethylenemalonate under catalyst- and solvent-free conditions, TetrahedronLetters, 2016, 57:321–324.
Related articles And Qustion
See also
Lastest Price from Diethyl ethoxymethylenemalonate manufacturers

US $0.00/kg2025-04-29
- CAS:
- 87-13-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-04-15
- CAS:
- 87-13-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg