What is the side effect of Nintedanib (BIBF-1120)
Description
Nintedanib (BIBF-1120), an orally administered intracellular tyrosine kinase inhibitor (TKI) initially developed as an antitumor agent, has antifibrotic properties and was one of the first drugs (along with the antifibrotic pirfenidone) to be approved for the treatment of IPF in the EU and USA; use of nintedanib in this indication has been previously reviewed in Drugs. More recently, nintedanib has been approved in the same regions for use in other chronic fibrosing ILDs with a progressive phenotype and SSc-ILD.
Pirfenidone & nintedanib
Pirfenidone and BIBF-1120 are antifibrotic medications approved for IPF treatment by regulatory agencies and available for clinical use worldwide. These drugs have been shown to reduce the rate of decline in forced vital capacity (FVC) and the risk of acute exacerbation among patients with IPF. Other ILDs may also show a progressive pulmonary fibrosis (PPF) phenotype. Recent data suggest that different ILDs with a PPF phenotype can share similar pathogenetic and biological pathways and could be amenable to the same treatment. Thus, it is biologically reasonable that pharmacological agents with antifibrotic properties, such as pirfenidone and nintedanib, may be efficacious in non-IPF PPF and fibrotic ILD. Unlike nintedanib, pirfenidone is approved only for treating IPF[1].
Side effects
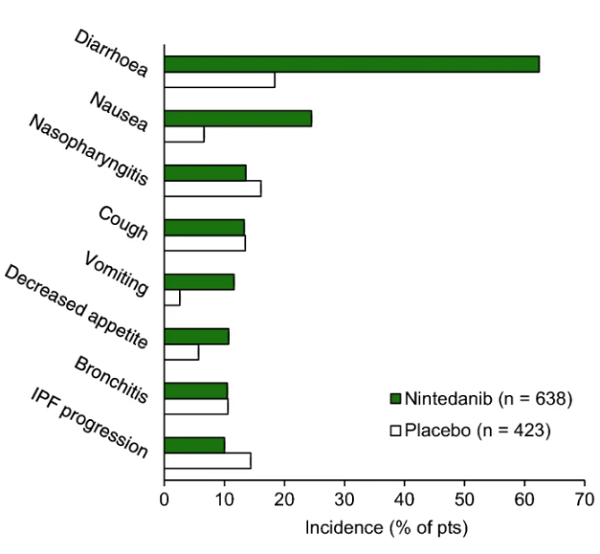
Gastrointestinal (GI) GI AEs were the most frequently reported AEs in clinical trials and global pharmacovigilance data. In the latter, diarrhoea had an incidence rate of 301.6 events/1000 PY and most diarrhea events were non-serious (97%). GI perforation was rare, occurring at a 1.0 event/1000 PY rate. Diarrhoea should be managed with adequate hydration and antidiarrhoeal medication (e.g., loperamide), and patients with nausea or vomiting should receive appropriate supportive care, including anti-emetic therapy if required. If GI AEs cannot be managed with supportive care, dosage modifications or treatment interruptions (or, in severe cases, treatment discontinuation) may be required[2].
Drug-induced liver injury, while uncommon, has been associated with BIBF-1120. In global pharmacovigilance data, elevated liver enzyme or bilirubin levels occurred at a rate of 31.5 events/1000 PY, with a median time to first onset of 60 days. Liver enzyme and bilirubin elevations were typically reversible with dose interruption or reduction in clinical trials. Certain patients may be at higher risk of elevations, including those female, Asian, or small physique. Liver function tests should be conducted before initiating treatment and at appropriate intervals; consult local prescribing information for further details.
VEGF receptor inhibition by nintedanib may increase the risk of bleeding. In pooled INPULSIS data, bleeding events were reported in 10.3% of nintedanib recipients versus 7.8% of placebo recipients. The incidence of severe bleeding events was similar between treatment groups (1.3% vs 1.4%). Bleeding occurred at a rate of 36.8 events/1000 PY in global pharmacovigilance data, with the majority of bleeding events being non-serious (81%) and the most frequently reported type of bleeding event being epistaxis (25% of events).
Arterial thromboembolic events have been infrequently reported with nintedanib. In the INPULSIS trials, cardiac disorder AEs occurred in 10.0% of nintedanib recipients (vs. 10.6% of placebo recipients), serious cardiac disorder AEs occurred in 5.0% (vs. 5.4%), and fatal cardiac disorders occurred in 0.5% (vs. 1.4%). Major cardiovascular AEs and myocardial infarction were reported at rates of 13.4 and 4.3 events/1000 PY in global pharmacovigilance data. Nintedanib use should be approached with caution in patients at higher cardiovascular risk [37, 51]. Treatment interruption should be considered if signs or symptoms of acute myocardial ischaemia develop.
References
[1] Francesco Amati. “Efficacy of Pirfenidone and Nintedanib in Interstitial Lung Diseases Other than Idiopathic Pulmonary Fibrosis: A Systematic Review.” International Journal of Molecular Sciences 24 9 (2023).
[2] Lamb, Yvette N. . "Nintedanib: A Review in Fibrotic Interstitial Lung Diseases (vol 81, pg 575, 2021)." Drugs: International Journal of Current Therapeutics and Applied Pharmacology Reviews, Featuring Evaluations on New Drugs, Review Articles on Drugs and Drug Therapy, and Drug Literature Abstracts 9(2021):81.
Related articles And Qustion
See also
Lastest Price from BIBF-1120 manufacturers

US $0.00/kg2025-04-02
- CAS:
- 928326-83-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $15.00-10.00/KG2021-07-13
- CAS:
- 928326-83-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton