When was sorafenib tosylate approved by the FDA?
On December 20, 2005, the Food and Drug Administration (FDA) granted regular marketing approval for sorafenib tosylate 200-mg tablets (Nexavar, BAY43-9006, Bayer Pharmaceuticals Corp., West Haven, CT and Onyx Pharmaceuticals Corp., Emeryville, CA), a new, small-molecular entity and oral multi-kinase inhibitor for the treatment of patients with advanced renal cell carcinoma (RCC). This indication is based on the demonstration of substantially improved progression-free survival (PFS) in a large, randomized, double-blinded, placebo-controlled phase 3 multinational study and a supportive phase 2 study. Overall survival results from the phase 3 study are preliminary.

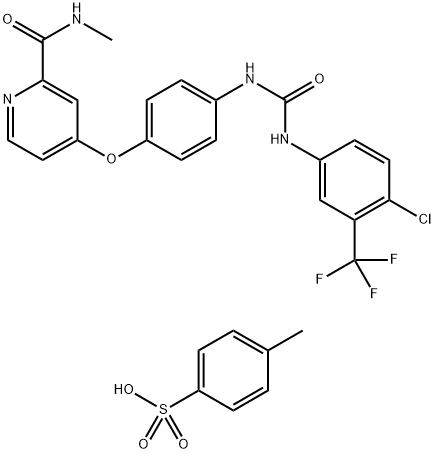
Sorafenib tosylate (the tosylate salt form), chemical name 4-(4-{3-[4-chloro-3-(trifluoromethyl)phenyl]ureido}phenoxy)-N2-methylpyridine-2-carboxamide-4-methylbenzenesulfonate, is a white to yellowish or brownish solid with a molecular formula of C21H16ClF3N4O3.C7H8O3S and a molecular weight of 637.03. Sorafenib tosylate is manufactured via chemical synthesis by coupling picolinamide phenyl ether, a key intermediate, with 4-chloro-3-trifluoromethylphenylisocyanate, followed by salt formation. Sorafenib tosylate is practically insoluble in aqueous media, slightly soluble in ethanol, and in PEG 400[1].
The USFDA has approved sorafenib tosylate for the treatment of thyroid cancer, kidney cancer, and hepatocellular carcinoma. Conventional formulations of sorafenib tosylate have challenges of solubility, permeability, side effects, and drug resistance across cancer cells[2]. Targeted cancer therapies based on nanotechnology have proven to be effective and have the potential to become a valuable tool in these approved cancer indications. In addition, Sorafenib tosylate's novel formulations are also being investigated for unapproved indications like hepatocellular cancer, renal cancer, and cholangiocarcinoma with promising outcomes. Nanotechnology-based formulations have shown considerable gains in bioavailability, absorption, and increased anticancer efficacy.
References
[1] Hrushikesh Raut . “Sorafenib tosylate novel drug delivery systems: Implications of nanotechnology in both approved and unapproved indications.” OpenNano 8 (2022): Article 100103.
[2] Robert C. Kane. “Sorafenib for the Treatment of Advanced Renal Cell Carcinoma.” Clin Cancer Res (2006) 12 (24): 7271–7278.
Related articles And Qustion
Lastest Price from Sorafenib tosylate manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 475207-59-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 475207-59-1
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 500KGS