Sorafenib Tosylate: Biological Activities and Future Developments
Sorafenib tosylate is a multi-target, multi-kinase inhibitor developed by Bayer in Germany. It was first approved by the US FDA in 2005 and is mainly used to treat adult liver cancer, thyroid cancer or kidney cancer.

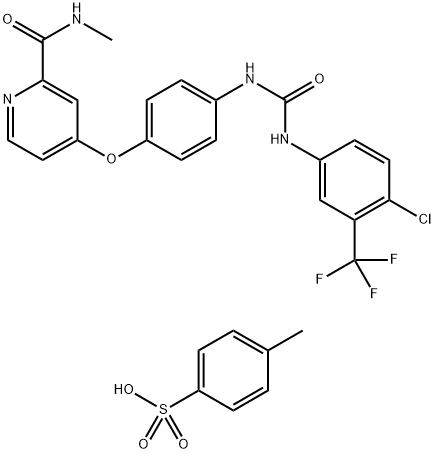
Figure 1. Sorafenib tosylate
Usage and Dosage
The common dose for adults is 400 mg, orally, twice a day. Duration of treatment: until the patient no longer benefits from treatment or unacceptable toxicity occurs. Note: The drug should be taken on an empty stomach, at least 1 hour before or 2 hours after a meal. It is recommended to swallow the tablet with a glass of water.
Adverse Reactions
The most common adverse reactions (≥20%) are diarrhea, fatigue, infection, hair loss, hand-foot skin reaction, rash, weight loss, decreased appetite, nausea, gastrointestinal and abdominal pain, hypertension and bleeding.
Contraindications
1. Patients with severe allergic reactions to Sorafenib tosylate or any inactive ingredients in this drug are contraindicated.
2. Sorafenib tosylate should not be used in combination with paclitaxel and carboplatin to treat patients with squamous cell lung cancer.
Sorafenib tosylate precautions
When using Sorafenib tosylate, the following are some important precautions:
1. Allergic reactions: Patients with a history of severe allergies to Sorafenib tosylate or its inactive ingredients should avoid using it.
2. Cardiac function: Sorafenib tosylate may affect cardiac function, so the patient's cardiac condition should be evaluated before use and monitored regularly during treatment.
3. Bleeding risk: Sorafenib tosylate may increase the risk of bleeding, especially in patients with a tendency to bleed or taking anticoagulants.
4. Hypertension: Sorafenib tosylate may cause or aggravate hypertension, so blood pressure needs to be monitored regularly and managed when necessary.
5. Skin toxicity: Sorafenib tosylate may cause skin reactions such as rash and hand-foot syndrome. Skin changes should be closely observed and appropriate skin care measures should be taken.
6. Effects on liver, kidney and thyroid function: Sorafenib tosylate may affect liver, kidney and thyroid function, so liver, kidney function and thyroid hormone levels should be checked and monitored regularly before and during treatment, and corresponding dose adjustments should be made in a timely manner.
7. Dietary restrictions: Avoid eating grapefruit or grapefruit juice during the use of Sorafenib tosylate as they may affect the metabolism of the drug.
Related articles And Qustion
See also
Lastest Price from Sorafenib tosylate manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 475207-59-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 475207-59-1
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 500KGS