Ammonium lauryl sulfate: Applications and Toxic Mechanism
General Description
Ammonium lauryl sulfate is a versatile compound with applications ranging from enhancing Proton Exchange Membrane Fuel Cells performance to the preparation of latex polystyrene, due to its effective emulsifying properties. In Proton Exchange Membrane Fuel Cells, Ammonium lauryl sulfate incorporation into gas diffusion layers significantly improves fuel cell efficiency, particularly under high humidity conditions, by optimizing gas diffusion and electrochemical performance. Similarly, in creating latex polystyrene, Ammonium lauryl sulfate stabilizes the emulsion, ensuring uniform particle distribution crucial for the material's properties. However, Ammonium lauryl sulfate has been associated with toxic effects, including apoptotic cell death through mitochondrial dysfunction, indicating potential health risks upon chronic exposure. This dual nature underscores the importance of careful management of Ammonium lauryl sulfate applications to harness its benefits while minimizing adverse effects.

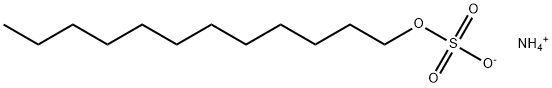
Figure 1. Ammonium lauryl sulfate
Applications
Fuel Cells
The microporous layer of the gas diffusion layers (GDLs) was fabricated with the carbon slurry dispersed in water containing ammonium lauryl sulfate (ALS) using the wire rod coating method and evaluated the effects on PEMFC performance. GDLs were also fabricated with the carbon slurry dispersed in water containing sodium dodecyl sulfate (SDS) and multiwalled carbon nanotubes (MWCNTs) with isopropyl alcohol (IPA) based for fuel cell performance comparison. Membrane electrode assemblies (MEA) with GDLs fabricated using MWCNTs and SDS exhibit the highest performance at 60 and 70% RH with a peak power density of 1100 and 850 mW.cm−2 using oxygen and air as an oxidant, respectively. This means that the gas diffusion characteristics of these two samples were optimum at 60 and 70% RH with extremely high limiting current density values. It was also found that the composition of the carbon slurry, specifically ammonium lauryl sulfate concentration has the highest peak power density of 1300 and 500 mW.cm−2 for both H2/O2 and H2/Air at 100% RH, respectively. However, SDS and MWCNTs based GDLs exhibited the lowest peak power density values using air as well as with oxygen as an oxidants at 100% RH.1
Preparation of Latex Polystyrene
Preparation of latex polystyrene with emulsifier ammonium lauryl sulfate (ALS) has been carried out by dissolving styrofoam (foam polystyrene) in toluene (30/70). And then polystyrene solution was mixed with aquadest, at ratios polystyrene and aquadest (v/v) were 90:10, 70:30, 50:50, 70:30, and 10:90 then added 10 mL of sodium lauryl sulfate (NLS) solution at concentrations 30%. The characterizations of the latex polystyrene included the stability determination during storage by density measurement and the determination of particle sizes and forms by microscopic optic observation. The results show that latex polystyrene ratio 90:10 is the most stable with density value constant during storage, are 0,848 g/mL for 30%. The particle sizes average is 1,56 mm with the distribution particle sizes decreasing as latex is 8,3 mm. Photomicrograph microscope optic shows that latex polystyrene NLS 30% has familiar particle forms and sizes.2
Potential Toxicity
A recent epidemiological study suggested that chronic exposure to cleaning detergents significantly reduced lung function in consumers. In this study, we identified the toxic mechanism of ammonium lauryl sulfate (ALS), the most common detergent in consumer products, using alveolar macrophage cells. In preliminary tests, cell viability sharply decreased between 40 and 200 μg/mL, thus we determined doses of 10, 20, and 50 μg/mL for further study. When treated at a 50 μg/mL for 24 h, cell viability was 67.7 ± 3.4% of the control, and autophagosome-like vacuoles and a number of double membranes surrounding damaged mitochondria were observed in the cytosol. Intracellular ROS, the ATP amount, ER volume, acid cell compartments and mitochondrial potential rapidly reduced with dose, whereas the release of LDH and apoptotic bodies dramatically increased. Additionally, multiple cell death pathways were activated following exposure to ALS, and the expression of caveolin-1, p-Acetyl CoA carboxylase, p21, and p-ERK were greatly inhibited. Moreover, the secretion of inflammatory mediators and expression of innate- and adaptive-immune response-related proteins were remarkably reduced. Meanwhile, the secretion of TGF-β was enhanced. Therefore, we conclude that ALS-induced apoptosis may be due to mitochondrial dysfunction triggered by the inhibition of caveolin-1, and that chronic pulmonary exposure to ALS may cause adverse health effects such as cancer and fibrosis by impairing the host's pulmonary immune system.3
References:
[1] R. VILLACORTA A M K. Development and Characterization of Gas Diffusion Layer Fabricated Using Carbon Slurry with Ammonium Lauryl Sulfate for Proton Exchange Member Fuel Cells[C]//59 10. 2012: 1159-1368. DOI:10.1002/jccs.201200367.[2] OSMAN H. The Effect of Variation of Latex Mixed Storage Time Polystyrene and Natural Rubber Concentrate Latex on the Stability of the Emulsion by Using Emulsifier Sodium Lauryl Sulfate[J]. Journal of Chemical Natural Resources, 2022, 19 1: 122110. DOI:10.32734/jcnar.v4i1.9354.
[3] EUN-JUNG PARK . Ammonium lauryl sulfate-induced apoptotic cell death may be due to mitochondrial dysfunction triggered by caveolin-1[J]. Toxicology in Vitro, 2019, 57: 1-254. DOI:10.1016/j.tiv.2019.02.021.
Related articles And Qustion
Lastest Price from Ammonium lauryl sulfate manufacturers

US $55.00/kg2025-04-21
- CAS:
- 2235-54-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $100.00-45.00/kg2025-04-15
- CAS:
- 2235-54-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton