Application research of Dibenzenesulfonimide and its derivative
Introduction
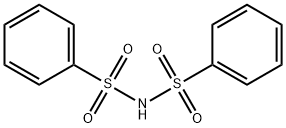
Dibenzenesulfonimide (Figure 1) is known as a weak acid with pKa of 1.45, which may be partially ionized to generate a proton ion and a dibenzenesulfonimide anion. The major species in chloroform may still be dibenzenesulfonimide. So that the reaction mechanism should be similar to that of X−H (X=Cl,F) bonds insertion. In previous study, dibenzenesulfonimide was selected as nitrogen source as its structure is similar to that of N-fluorobenzenesulfonimide(NFSI),which resulted in a rapid formation of α-amino-ester derivative.[1]
[
Amination of Diazocarbonyl Compounds
Transition-metal-free intermolecular N-H insertion of α-diazocarbonyl compounds is reported. Among the series of nitrogen sources examined, dibenzenesulfonimide was found to be the choice in terms of the yields and the reaction time. Primary mechanistic experiments suggest that a pathway involving a sequence of protonation and nucleophilic substitution was preferred.[1]
N-Thiocyanato-Dibenzenesulfonimide
A novel electrophilic thiocyanating reagent, N-thiocyanato-dibenzenesulfonimide exhibited enhanced electrophilicity with a wide scope of substrates. Numerous electrophilic thiocyano oxyfunctionalization reactions of alkenes have been achieved using N-thiocyano-dibenzenesulfonimide, which is a new electrophilic thiocyanation reagent and could be easily prepared in two steps from dibenzenesulfonimide. This approach provides efficient, simple, and modular methods for the formation of SCN-containing heterocycles such as lactones, tetrahydrofurans, dihydrofurans, and dihydrobenzofurans in moderate to excellent yields. Meanwhile, diverse oxa-quaternary centers were rapidly constructed. Additionally, this protocol is free of transition metals and features broad substrate toleraance and mild reaction conditions.[2]
Thus, it reacted with activated aromatics such as phenols, indoles, anilines and anisoles without a catalyst giving the corresponding thicyanate derivatives in high yields, while TfOH for unactivated arenes and hetero aromatics and Zn(OTf)2 for ketones was used as the catalyst, respectively. It is noteworthy that internal alkenes and styrenes were bifunctionalized giving 1,2-amino thiocyanates in high yields.[3] Furthermore,a highly regioselective protocol for intermolecular thiocyanation-amination of alkynes by N-thiocyano-dibenzenesulfonimide as the SCN and nitrogen sources has been developed. A C-S bond and C-N bond are simultaneously constructed in only one step. The reaction under simple mild conditions features a broad substrate scope, atom economy, high yields (up to 94%), and excellent functional group tolerance.[4]
N-Trifluoromethylthio-dibenzenesulfonimide
To verify the theoretical prediction, zhang et al. set to synthesize N-trifluoromethylthio-dibenzenesulfonimide and test its reactivity. The preparation of N-trifluoromethylthio-dibenzenesulfonimide was relatively easy and can be accomplished in two steps starting from dibenzenesulfonimide. However, the purification of this compound is a rather tedious task. Treatment of bis(phenylsulfonyl)imide with tert-butyl hypochlorite in methanol at room temperature for 5 min generated N-chlorodibenzenesulfonimide in 73% yield, which was further reactedwith 1.2 equiv of AgSCF3 in CH2Cl2 for 2 h to form N-trifluoromethylthio-dibenzenesulfonimide in 89% yield, as determined by 19F NMR spectroscopy. Consistent with the theoretical prediction, N-trifluoromethylthio-dibenzenesulfonimide exhibits reactivity remarkably higher than that of other known electrophilic trifluoromethylthiolating reagents. In the absence of any additive, N-trifluoromethylthio-dibenzenesulfonimide reacted with a wide range of electron-rich arenes and activated heteroarenes under mild conditions. Likewise, reactions of N-trifluoromethylthio-dibenzenesulfonimide with styrene derivatives can be fine-tuned by simply changing the reaction solvents to generate trifluoromethylthiolated styrenes or oxo-trifluoromethylthio or amino-trifluoromethylthio difunctionalized compounds in high yields.[5]
Synthesis of difluoromethylated allenes
Organofluorine compounds have shown their great value in many aspects. Moreover, allenes are also a class of important compounds. Fluorinated or fluoroalkylated allenes might provide an option as candidates for drug and material developments, as allenes allow a great number of valuable transformations. Herein, Taj Muhammad M et al. Had reported a metal-free synthesis of difluoromethylated allenes via regioselective trifunctionalization of 1,3-enynes. This method proceeds through double C-F bond formation with concomitant introduction of an amino group to the allene. Synthetic applications are conducted and preliminary mechanistic studies suggest that a two-step pathway is involved. DFT calculations revealed an unusual dibenzenesulfonimide-assisted fluorination/fluoroamination with NFSI. In addition, kinetic reaction study revealed the induction period of both major and side products to support the proposed reaction mechanism. This work offers a convenient approach for the synthesis of a range of difluoromethylated allenes and is also a rare example of trifunctionalization of 1,3-enynes.[6]
References
[1] Luo X, Chen G, He L, Huang X. Amination of Diazocarbonyl Compounds: N-H Insertion under Metal-Free Conditions. J Org Chem. 2016;81(7):2943-2949. doi:10.1021/acs.joc.6b00233
[2] Ye AH, Zhang Y, Xie YY, et al. TMSCl-Catalyzed Electrophilic Thiocyano Oxyfunctionalization of Alkenes Using N-Thiocyano-dibenzenesulfonimide. Org Lett. 2019;21(13):5106-5110. doi:10.1021/acs.orglett.9b01706
[3] Li C, Long P, Wu H, Yin H, Chen FX. N-Thiocyanato-dibenzenesulfonimide: a new electrophilic thiocyanating reagent with enhanced reactivity. Org Biomol Chem. 2019;17(30):7131-7134. doi:10.1039/c9ob01340g
[4] Wu H, Shao C, Wu D, Jiang L, Yin H, Chen FX. Atom-Economical Thiocyanation-Amination of Alkynes with N-Thiocyanato-Dibenzenesulfonimide. J Org Chem. 2021;86(7):5327-5335. doi:10.1021/acs.joc.0c02780
[5] Zhang P, Li M, Xue XS, et al. N-Trifluoromethylthio-dibenzenesulfonimide: A Shelf-Stable, Broadly Applicable Electrophilic Trifluoromethylthiolating Reagent. J Org Chem. 2016;81(17):7486-7509. doi:10.1021/acs.joc.6b01178
[6] Taj Muhammad M, Jiao Y, Ye C, et al. Synthesis of difluoromethylated allenes through trifunctionalization of 1,3-enynes. Nat Commun. 2020;11(1):416. Published 2020 Jan 21. doi:10.1038/s41467-019-14254-3
See also
Lastest Price from Dibenzenesulfonimide manufacturers
US $1.00/kg2025-04-21
- CAS:
- 2618-96-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/KG2025-04-15
- CAS:
- 2618-96-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg