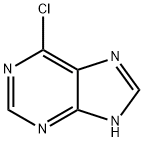
6-Chloropurine: Antiviral Nucleoside Scaffold and Synthetic Building Block
6-Chloropurine is a building block in chemical synthesis. Intermediate in the preparation of 9-alkylpurines and 6-rnercaptopurine. 6-Chloropurine to S-(6-punnyl)glutathione and further metabolism of S-(6-punnyl)glutathione to 6-mercaptopunne may be involved in the mechanism of the antitumor activity. It has been shown to have synergic antitumor properties in a variety of mouse neoplasms when administered simultaneously.

Nucleoside analogues having 6-chloropurine as anti-SARS-CoV agents
Severe acute respiratory syndrome (SARS) is an emerging infectious disease of the 21st century and poses a global threat to public health, mainly leading to fatal infection with influenza-like symptoms such as high fever, dry cough, pneumonia and dyspnoea. This disease appeared in the Guandong province of southern China in late 2002, and subsequently spread to 29 countries in early 2003, affecting approximately 8000 persons. In our previous studies, several nucleoside derivatives having 6-chloropurine as the nucleobase showed potent antiviral activity against some types of viruses. We presume that the moiety could play an important role in the antiviral activity; in fact, several 6-chloropurine analogues are known to inhibit bacterial RNA polymerases. Therefore, we expected that nucleoside analogues that have 6-chloropurine would be efficacious against SARS-CoV. Thus, several nucleoside analogues, which could be readily prepared or are commercially available, were designed as anti-SARS-CoV agents.[1]
A chlorine atom at the 6-position of the purine base was considered to be important for the anti-SARS-CoV activity; however, an introduction of the amino group at the 2-position of 6-chloropurine, which corresponds to a guanine derivative, was unfavourable for the antiviral activity. Although the reason for this trend is unclear, there is a possibility that due to its electrophilic nature, the moiety can form a covalent bond with the target enzyme and can induce an effective irreversible inhibition. In conclusion, we have synthesized several nucleoside analogues having 6-chloropurine as the nucleobase. Among these analogues, two compounds, were found to be efficacious against SARS-CoV and showed antiviral activities comparable to those of mizoribine and ribavirin. This study revealed several curious SAR trends such as the antiviral effects of the 6-chloropurine moiety, unprotected 5′-hydroxyl group, and protected (benzoylated) 5′-hydroxyl group.
New antitumor 6-chloropurine nucleosides
Purine nucleosides and nucleotides have been major targets of anticancer research for several decades. One of the first investigations was accomplished by the group of Noell in 1962 synthesizing a series of thioguanines and 2-amino-6-alkylthiopurine derivatives. Caba et al. reported on an antiproliferative agent against the MCF-7 adenocarcinoma with micromolar GI50 value embodying tetrahydrobenzoxazepine N9-linked to a 6-chloropurine while a coumarin N9-linked to a 2-amino-6-chloropurine showed moderate activity (25–35 μM) against the HeLa, HepG2 and SW620 cell lines. The previously reported anticancer potential of 6-chloropurine derived compounds encouraged us to investigate, for the first time, a series of purines embodying a 6-chloro substitution (CP) or both 6-chloro- and 2-acetamido groups (ACP), linked at N7 or N9 to perbenzylated d-glucosyl, d-mannosyl and d-galactosyl residues. The reaction conditions described by Marcelo et al. using a silylated base was optimized for this type of nucleosides. The anticancer activity was determined using a sulforhodamine B (SRB) assay to yield GI50 values for human melanoma, lung and ovarian carcinoma, and colon adenocarcinoma cancer cell lines.[2]
Treating cancer has been challenging for decades, following countless approaches and attempts. Nucleosides, alone or as part of nucleotides, are vital elements of living systems and have shown pharmacological effects, e.g. as antibiotic or antiviral agents. We investigated the antitumor potential on human melanoma, lung and ovarian carcinomas, and on colon adenocarcinoma of a new series of purine nucleosides based on a 6-chloropurine or a 2-acetamido-6-chloropurine scaffold linked to perbenzylated hexosyl (glucosyl, galactosyl and mannosyl) residues. All compounds were tested in a sulforhodamine B (SRB) assay for their cytotoxicity and provided micromolar GI50 values with order of magnitude comparable to structurally similar chemotherapeutics, namely 2-chloro-2′-deoxyadenosine (cladribine). Furthermore, the induction of apoptosis was established and cell cycle analysis was accomplished demonstrating a G2/M cell cycle arrest.
References
[1]Ikejiri M, Saijo M, Morikawa S, Fukushi S, Mizutani T, Kurane I, Maruyama T. Synthesis and biological evaluation of nucleoside analogues having 6-chloropurine as anti-SARS-CoV agents. Bioorg Med Chem Lett. 2007 May 1;17(9):2470-3. doi: 10.1016/j.bmcl.2007.02.026. Epub 2007 Feb 13. PMID: 17336519; PMCID: PMC7126875.
[2]Schwarz, Stefan et al. “New antitumor 6-chloropurine nucleosides inducing apoptosis and G2/M cell cycle arrest.” European journal of medicinal chemistry vol. 90 (2015): 595-602. doi:10.1016/j.ejmech.2014.11.019
Lastest Price from 6-Chloropurine manufacturers
US $0.00/kg2025-08-26
- CAS:
- 87-42-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $10.00/ASSAYS2025-08-19
- CAS:
- 87-42-3
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton