ChemicalBook > Articles Catagory List >请选择分类 >what-is-the-most-direct-laboratory-method-for-synthesizing-methyl-isonicotinate
What is the most direct laboratory method for synthesizing Methyl Isonicotinate?
Nov 27,2025
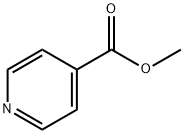
The most direct and widely used laboratory method for synthesizing isonicotinic acid methyl ester is the Fischer esterification reaction of its parent compound, isonicotinic acid (pyridine-4-carboxylic acid). In this reaction, the carboxylic acid reacts directly with methanol in the presence of a strong acid catalyst (usually concentrated sulfuric acid or thionyl chloride). The specific steps are as follows: isonicotinic acid is suspended in excess methanol, the mixture is cooled, and then the acid catalyst is slowly added to shift the equilibrium towards the esterification product. Finally, the mixture is heated to reflux to complete the reaction. This is a classic, high-yield method for converting pyridine carboxylic acids to the corresponding methyl ester. Alternatively, the ester can be prepared by oxidizing 4-methylpyridine to isonicotinic acid followed by esterification, or by transesterification of other alkyl isonicotinic acid esters.
You may like
What is 9-fluorenol used for?
Dec 19, 2025
Lastest Price from Methyl isonicotinate manufacturers
Methyl isonicotinate
US $0.00-0.00/kg2025-11-19
- CAS:
- 2459-09-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons
Methyl isonicotinate

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 2459-09-8
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 300/month