Chemical Properties
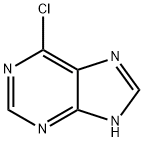
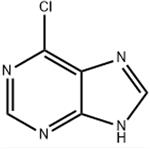
It is a pale yellow crystal with relatively stable chemical properties that do not cause decomposition at room temperature. Its structure includes nitrogen heterocycles, which give rise to remarkable alkalinity and the formation of volatile acid salts. It is soluble in dimethyl sulfoxide, but has only slight solubility in water.
Uses
6-Chloropurine is used in the preparation of 9-alkylpurines through alkylation with various substituted alkyl halides in dimethyl sulfoxide. It is also used to prepare 6-succinoaminopurine.
Definition
ChEBI: 6-Chloro-1H-purine is a member of purines. It is metabolized to form 6-mercaptopurine and has been used as an antineoplastic.
Preparation
6-chloropurine is synthesized by reacting hypoxanthine with phosphoryl chloride in the presence of an unreactive base such as dimethyl aniline. It may likewise be formed by heating hypoxanthine under pressure with phosphoryl chloride as well as with a reagent prepared by adding water, preferably about by volume, to dry phosphoryl chloride.
US2832781A
Synthesis Reference(s)
Journal of the American Chemical Society, 78, p. 1928, 1956
DOI: 10.1021/ja01590a043
General Description
The acid-catalyzed reaction of 6-chloropurine with 3,4-di-
O-acetyl-D-xylal has been investigated.
Purification Methods
6-Chloropurine crystallises from water. The UV in water at pH 1 has 264nm (log 3.94). [Beilstein 26 III/IV 1742.]