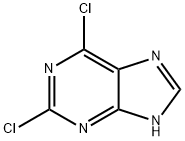
Description
2,6-Dichloropurine is an important pharmaceutical intermediate. It is widely used in the preparation of purine nucleosides and purine nucleotides
[1].
Chemical Properties
white to light yellow crystal powder
Uses
2,6-Dichloropurine is used in the synthesis of 2,6-diamino-substituted purine derivatives as potential cardiomyogenesis inducing agents.
Uses
Suzuki-Miyaura cross-coupling between halopurines and arylboronic acids in water-acetonitrile.1
Synthesis Reference(s)
Journal of the American Chemical Society, 80, p. 404, 1958
DOI: 10.1021/ja01535a040
Synthesis
2,6-Dichloropurine is prepared in two main ways:
(1) By chlorination of the purine ring structure, e.g., chlorination of xanthine (2,6-dihydroxypurine) with pyrophosphoryl chloride at high temperatures in sealed tubes in the presence of a phase-transfer catalyst or with phosphorus oxychloride under reflux, and chlorination of 6-chloropurine, hypoxanthine, or their N-oxides with phosphorus oxychloride, and chlorination with chlorine gas at low temperatures. Chlorination of 2,6-dithiopurine.
(2) The purine ring is constructed using barbituric acid derivatives or 2,4-dichloro-5,6-diaminopyridine as starting materials. However, both methods are not very suitable for industrial production.
The industrial preparation of 2,6-dichloropurine involves the direct chlorination of xanthine with phosphorus trichloride and a weakly nucleophilic organic base (e.g., amidine, guanidine base, or proton sponge)
[1]. The reaction process is shown below:
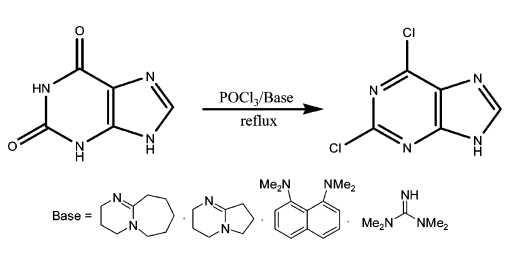
Purification Methods
It can be recrystallised from 150 parts of boiling H2O and dried at 100o to constant weight. It is soluble in EtOAc. The HgCl2 salt separates from EtOH solution. UV: max 275nm ( 8.9K) at pH 1; and 280nm ( 8.5K) at pH 11 [Elion & Hitchings J Am Chem Soc 78 3508 1956, Schaeffer & Thomas J Am Chem Soc 80 3738 1958, Beaman & Robins J Appl Chem (London) 12 432 1962, Montgomery J Am Chem Soc 78 1928 1956]. [Beilstein 26 III/IV 1747.]
References
[1] QI ZENG. Facile and Practical Synthesis of 2,6-Dichloropurine[J]. Organic Process Research & Development, 2004, 8 6: 962-963. DOI:10.1021/op049878r.