Ammoniacal Leaching and Oxidation of Ammonium Sulfite
Introduction
With the rapid expansion of electric vehicles and electronic devices, the recycling of spent lithium-ion batteries (LIBs) has become increasingly important for recovering valuable metals such as cobalt (Co), nickel (Ni), and lithium (Li). Hydrometallurgical processes, known for their selectivity and environmental advantages, have been explored as sustainable alternatives to conventional pyrometallurgical methods. Among these, ammonium-based leaching systems are particularly promising because they utilize ammonia and its derivatives to form soluble complex ions that enhance metal dissolution. Notably, ammonium sulfite plays a dual role: it acts as a reductant in metal leaching and is also a key compound in industrial processes for pollution control. In power plants, aqueous ammonia is often used to scrub flue gases and remove sulfur dioxide (SO₂), producing ammonium sulfite as a byproduct, which can be easily oxidized to ammonium sulfate—a valuable fertilizer. Therefore, the application of ammonium sulfite in hydrometallurgical recycling not only supports efficient recovery of critical metals from LIBs but also connects with flue gas desulfurization technology, providing both environmental and industrial benefits.
Reductive Ammonia Leaching of Metals from Cathode Scrap by Sodium Sulphite
Recently a work demonstrated a hydrometallurgical process that can selectively leaching Co, Ni, and Li from the hybrid electrode power of a lithium-ion battery using a ternary ammoniacal solution which contains ammonia, ammonium sulfite, and ammonium bicarbonate. The leaching kinetics of valuable metals in the ammobiacal solution are dependent on ammonium sulfite, which is necessary as a reductant. Using favorable conditions for soluble ammonia-complex ion formation is advantageous for enhancing the leaching efficiency of Li, Ni, and Co. under optimum conditions with 1.5-M ammonia, 1-M ammonium sulfate, and 1-M ammonium bicarbonate at 60℃, a pulp density of 20g/L, and leaching time of 180 min, Ni and Cu could be almost completely leached out, and the leaching efficiencies of Li and Co were 60.53% and 80.99%. However, very little Al leached out, regardless of charges in the leaching conditions, and the leaching efficiency is negligible owing to Al’s low content in the cathode power. [1]
Rete of Ammonium Sulfite Oxidation in Aqueous Solutions
The homogeneous oxidation of ammonium sulfite in aqueous solution was studied by batchwise experiment. The conversion of sulfite was roughly proportional to the period of time of bubbling gaseous oxygen into a solution. Also, the absorption rate of gaseous oxygen increased linearly with the sulfite concentration, but beyond a certain value of the concentration the absorption rate decreased slightly with increase in the sulfite concentration. A qualitative experiment on the absorption rates of oxygen into sulfurous acid solutions with various co-existing ions by a mano-metric measurement reported that the absorption rate into a sodium sulfite solution was larger than that into an ammonium sulfite solution. A study also found the rate of the homogeneous reaction of dissolved oxygen and ammonium sulfite in aqueous solutions without catalysts. The existence of inhibitors suggested that the reaction might be a radical one. An empirical rate equation, r=k[SO32-]3/2[O2]0[H+]2 in M•min-1 with k= 1.6× 1039exp(-35× 103/RT), was obtained in the experimental range of this work. [2]
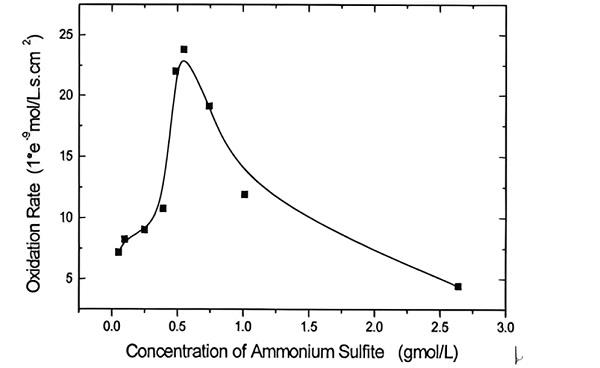
Kinetics of Heterogeneous Oxidation of Concentrated Ammonium Sulfite
The heterogeneous oxidation of ammonium sulfite solution was investigated in stirred cell and packing column. The concentration range of sulfite is 0.05–4.7gmol/l and that of oxygen in the gas phase is 21–100%, temperature range is 30–75°C. The results indicated that there is a critical sulfite concentration, below which the reaction order with respect to sulfite is 0.2, while above which the order turns to −1.0. The reaction order with respect to oxygen is 1. Apparently, the ionic strength was found to inhibit the oxidation. The apparent energy of activation determined is 32.7kJ/mol. Based on the data observed in Fig. 1, the order of reaction with respect to sulfite is 0.2 below the critical concentration and −1.0 above the critical concentration. The kinetics of oxidation of concentrated ammonium sulfite can be expressed as R=713e-3.27e4/RTPO2[SO32-]-1(1-0.425[SO24-]). [3]
References:
[1] Wu, C., Li, B., Yuan, C., Ni, S., & Li, L. (2019). Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Management, 93, 153-161.
[2] Matsuura, A., Harada, J., Akehata, T., & Shirai, T. (1969). Rate of ammonium sulfite oxidation in aqueous solutions. Journal of Chemical Engineering of Japan, 2(2), 199-203.
[3] Zhou, J., Li, W., & Xiao, W. (2000). Kinetics of heterogeneous oxidation of concentrated ammonium sulfite. Chemical engineering science, 55(23), 5637-5641.
You may like
Lastest Price from Ammonium Sulfite manufacturers

US $9.00/kg2025-04-21
- CAS:
- 10196-04-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $50.00/kg2025-04-21
- CAS:
- 10196-04-0
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 5000