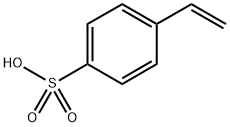
Application research of polystyrene sulfonic acid
Introduction
Polystyrene sulfonic acid (PSSA;Figure 1) ,a negatively charged polymer, is currently widely used in ion exchanging and proton conducting membranes, and sodium or calcium salts have been administered to humans to treat hyperkalemia via a potassium binding mechanism. However, it is rarely used in pharmaceutical formulations, possibly due to potential gastrointestinal side effects, limited safety data, and lack of historical pharmaceutical usage. Van Eerdenbrugh and Taylor have shown that polystyrene sulfonic acid can inhibit crystallization of several compounds and have studied its acid−base interactions with model compounds by using Fourier transform infrared spectroscopy (FTIR).[1]

Acid-Base Interactions of Polystyrene Sulfonic Acid in Amorphous Solid Dispersions
This study investigates the potential drug-excipient interactions of polystyrene sulfonic acid (PSSA) and two weakly basic anticancer drugs, lapatinib (LB) and gefitinib (GB), in amorphous solid dispersions. Based on the strong acidity of the sulfonic acid functional group, polystyrene sulfonic acid was hypothesized to exhibit specific intermolecular acid-base interactions with both model basic drugs. Ultraviolet (UV) spectroscopy identified red shifts, which correlated well with the color change observed in lapatinib- polystyrene sulfonic acid solutions. Fourier transform infrared (FTIR) spectra suggest the protonation of the quinazoline nitrogen atom in both model compounds, which agrees well with data from the crystalline ditosylate salt of lapatinib. X-ray photoelectron spectroscopy (XPS) detected increases in binding energy of the basic nitrogen atoms in both lapatinib and gefitinib, strongly indicating protonation of these nitrogen atoms. (15)N solid-state NMR spectroscopy provided direct spectroscopic evidence for protonation of the quinazoline nitrogen atoms in both LB and GB, as well as the secondary amine nitrogen atom in LB and the tertiary amine nitrogen atom in GB. The observed chemical shifts in the LB-polystyrene sulfonic acid (15)N spectrum also agree very well with the lapatinib ditosylate salt where proton transfer is known. Additionally, the dissolution and physical stability behaviors of both amorphous solid dispersions were examined. Polystyrene sulfonic acid was found to significantly improve the dissolution of LB and GB and effectively inhibit the crystallization of LB and GB under accelerated storage conditions due to the beneficial strong intermolecular acid-base interaction between the sulfonic acid groups and basic nitrogen centers.[1]
Low-fouling material preparation
A negatively charged hydrophilic low fouling film was prepared by thermally cross-linking a blend consisting of polystyrene sulfonic acid (PSSA) and polyethylene glycol (PEG). The film was found to be stable by dip-washing. The fouling resistance of this material toward bacterial (Escherichia coli) and colloidal (polystyrene particles) attachment, non-specific protein (fibronectin) adsorption and cell (3T3 NIH) adhesion was evaluated and was compared with glass slides modified with polyethylene glycol (PEG) brushes, oxidized 3-mercaptopropyltrimethoxysilane (sulfonic acid, SA), and n-octadecyltrichlorosilane (OTS). The extended Derjaguin-Landau-Verwey-Overbeek (XDLVO) theory and thermodynamic models based on surface energy were used to explain the interaction behaviors of E. coli/polystyrene particles-substrate and protein-substrate interactions, respectively. The cross-linked polystyrene sulfonic acid-PEG film was found to be slightly better than SA and PEG toward resisting non-specific protein adsorption, and showed comparable low attachment results as those of PEG toward particle, bacterial and NIH-3T3 cells adhesion. The low-fouling performance of polystyrene sulfonic acid -PEG, a cross-linked film by a simple thermal curing process, could allow this material to be used for applications in aqueous environments, where most low fouling hydrophilic polymers, such as polystyrene sulfonic acid or PEG, could not be easily retained.[2]
Magnetic nanoparticles coated with polystyrene sulfonic acid
The use of magnetic nanoparticles (MNPs) magnetized on applying an alternating magnetic field (AMF) to stimulate the thermal characteristics and to induce tumor apoptosis is a currently active area of research in cancer treatment. In previous work, we developed biocompatible and superparamagnetic polystyrene sulfonic acid-coated magnetic nanoparticles (PSSA-MNPs) as applications for magnetically labeled cell trapping, but without assessment of treatment effects on tumor diseases. In the present work, researchers examined PSSA-MNP-induced magnetic fluid hyperthermia (MFH) on SK-Hep1 hepatocellular carcinoma (HCC) cells for lethal thermal effects with a self-made AMF system; an adjustable AMF frequency generated a variable intensity of magnetic field and induced MNP relaxation. The extracellular and intracellular MFH treatments on a SK-Hep1 cell line were implemented in vitro; the result indicates that the lethal effects were efficient and caused a significantly decreased cell viability of SK-Hep1 cells. As the polystyrene sulfonic acid-MNP concentration decreased, especially in intracellular MFH treatments, the MFH effects on cells, however, largely decreased through heat spreading to the culture medium. On controlling and decreasing the volume of culture medium, the problem of heat spreading was solved. It can be consequently expected that polystyrene sulfonic acid-MNPs would be a prospective agent for intracellular cancer magnetotherapy.[3]
References
1.Song Y, Zemlyanov D, Chen X, et al. Acid-Base Interactions of Polystyrene Sulfonic Acid in Amorphous Solid Dispersions Using a Combined UV/FTIR/XPS/ssNMR Study. Mol Pharm. 2016;13(2):483-492. doi:10.1021/acs.molpharmaceut.5b00708
2. Alghunaim A, Zhang Newby BM. Cross-linked polystyrene sulfonic acid and polyethylene glycol as a low-fouling material. Colloids Surf B Biointerfaces. 2016;140:514-522. doi:10.1016/j.colsurfb.2016.01.026
3.Chen BW, Chiu GW, He YC, et al. Extracellular and intracellular intermittent magnetic-fluid hyperthermia treatment of SK-Hep1 hepatocellular carcinoma cells based on magnetic nanoparticles coated with polystyrene sulfonic acid. PLoS One. 2021;16(2):e0245286. Published 2021 Feb 5. doi:10.1371/journal.pone.0245286
See also
Lastest Price from POLYSTYRENE SULFONIC ACID manufacturers
US $0.00-0.00/kg2025-11-19
- CAS:
- 28210-41-5
- Min. Order:
- 1kg
- Purity:
- 29 wt%-31 wt%
- Supply Ability:
- 100kg

US $0.00/KG2025-04-21
- CAS:
- 28210-41-5
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month