5-Amino-o-cresol: Hair Dye Coupling Agent & Safety Assessment
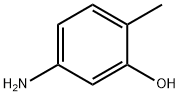
5-Amino-o-cresol is a kind of off-white powder with the CAS number 2835-95-2. It is a llittle soluble in water. It is mainly used as a coupling agent for oxidizing hair dyes, reacting with color developers to form natural hair color. As a key coupling component in hair dyeing formulas, 5-Amino-o-cresol can react with chromogenic agents (such as para-phenylenediamines and m-phenylenediamines) under the action of oxidants to generate natural pigments such as brownish yellow, light brown, and dark brown that are close to the original hair color. At the same time, it can enhance the uniformity and durability of the dyed color, reduce the risk of fading, and is widely compatible with home hair dyes, professional hair dyes for salons, and other products.

Contact sensitizing potential of pyrogallol and 5-amino-o-cresol in female BALB/c Mice
It is estimated that one in three women above the age of 18 and one in ten men above the age of 40 use some type of hair coloring. Permanent coloring accounts for approximately 70% of the dollar volume of the retail market and 85% of the professional market for hair care products. 5-amino-o-cresol (AOC) is an oxidative intermediate used in hydrogen peroxide-induced coupling reactions in permanent hair dye formulations. According to the Food and Drug Administration (FDA) product formulation data, 5-Amino-o-cresol was used in 149 out of 960 hair dye formulations in 1986, and it is still being used worldwide. AOC concentrations in hair dyes range between 0 – 5%. According to a study by Beck et al. , both pig skin (in vitro) and rat skin are permeable to 5-Amino-o-cresol. The objective of the present study was to evaluate the contact sensitization potential of both PYR and AOC when applied dermally to female BALB/c mice using the well-established local lymph node assay (LLNA), the irritancy assay (IRR), and the mouse ear swelling test (MEST). In addition to the LLNA and MEST, additional information on the sensitization potential of a compound can be obtained from flow cytometric analysis of the draining lymph nodes.[1]
In this study, 5-Amino-o-cresol induced a borderline positive response in only one of two LLNA studies conducted, and it failed to induce a positive MEST response. Thus, the contact sensitizing potential of AOC at concentrations up to 10% is considered to be minimal. In the report from the Scientific Committee on Consumer Products; Opinion on: 4-Amino-2-hydroxytoluene (Opinions SCCP, 2006), two murine studies using strain CBA/Ca01aHsd have been included regarding the skin sensitization potential of 5-Amino-o-cresol. In the first study, an EC3 of 0.44% was obtained when aqua/acetone (1:1) mixed with olive oil at a ratio of 4:1 was used as the vehicle. In the second murine study, an EC3 value of 3.4% was obtained when DMSO was used as vehicle. In our studies, an approximate EC3 value of 9% for AOC was obtained when the positive LLNA study was used for extrapolation. The vehicle, acetone:olive oil (4:1), might contribute partially to the discrepancy; however, the strain of mice used might play a major role because BALB/c is Th2-biased while CBA is Th1-biased (De Vooght et al., 2010). In summary, PYR, but not 5-Amino-o-cresol, elicited dermal sensitization in female BALB/c mice, as demonstrated using the LLNA and the MEST.
Determination of 5-amino-o-cresol and metabolites in human keratinocytes
In recent years, concern has arisen over the extensive use of synthetic colours, especially in cosmetics including hair dyes. Prediction of metabolic pathways of hair dyes after their application is an important aspect to meet international safety requirements of cosmetic products as formation of inactive metabolites as well as toxic compounds is likely. However, metabolism of the dyes used in this study has not been investigated previously. Therefore, the aim of this work was to develop an HPLC method for the separation of the two oxidation hair dyes 4-AC and 5-Amino-o-cresol and their metabolites formed in the alternative testing system cell culture using human keratinocytes (HaCaT) by HPLC/DAD and following identification by MS detection. Prediction of metabolites to be expected for 4-AC and 5-AC were carried out using the METEORreport software. As calculated by the software, the scheme is the same for 4-AC and 5-Amino-o-cresol corresponding to the position of the functional groups.[2]
A cell culture from human keratinocytes was selected as a medium in order to investigate biotransformation of the two oxidation hair dyes 4-AC and 5-Amino-o-cresol. The system used may best simulate the enzymatic situation being present on head skin after application of these aminocresols. To predict possible metabolites in biological material a special computer software was applied. For the separation of the two oxidation hair dyes 4-AC and 5-AC and their metabolites formed in the cell culture, numerous mobile phases were tested in the isocratic mode. However, a multi-step gradient system consisting of ACN–0.01 M acetate buffer pH 8.7 for 4-AC and metabolites and MeOH–0.01M acetate buffer pH 8.7 for 5-Amino-o-cresol and metabolites could be developed. Stationary phase was a hybrid-based RP8 column which allows pH higher than 8 in mobile phase. With MS analysis performed under optimized chromatographic conditions only one metabolite, the N-acetylated derivative, for each compound could be identified.
Toxicity Studies of 5-Amino-o-cresol
5-Amino-o-cresol is the most widely used member of a class of 25 chemicals, the aminocresols and related compounds, defined as amino- and methyl-substituted phenols, their salts, and ethers. Absorption of 5-amino-o-cresol was complete following gavage administration in rats (4 to 357 mg/kg) and mice (36 mg/kg). Excretion was mainly in the urine, and more than 80% of the administered dose was excreted in the urine within 24 hours. Absorption of 5-amino-o-cresol in rats 24 or 72 hours following dermal application (protected from oral grooming) was less than 10% of the applied dose (2.5 or 26 mg/kg; the application site was rinsed 6 hours after application). When the dermal site was unprotected from grooming, 36% to 66% of the applied dose (2.5 to 32 mg/kg) was absorbed within 24 or 72 hours indicating that the oral exposure through grooming contributes significantly to the total absorbed dose. As observed following oral or intravenous administration, the majority of the absorbed dose was excreted in the urine. The radioactivity in tissues was low (0.2% to 3.6% of the dose), regardless of the dose, route, or species.[3]
The Hazardous Substances Data Bank1 summarizes the results of several 5-amino-o-cresol studies previously published elsewhere. The acute 5-amino-o-cresol oral LD50 values are 3,600 mg/kg for CFY rats and 2,928 and 4,355 mg/kg for female and male Sprague-Dawley rats, respectively. A dermal dose of up to 5 g/kg produced no dermal toxicity in rabbits. Dietary administration of 5-amino-o-cresol in subchronic toxicity studies in Sprague Dawley rats was conducted at concentrations of 0%, 0.3%, 1%, or 3% for periods of 3 to 6 months. Significant reductions in body weights occurred in male and female rats fed 3% and in males fed 1%. No significant differences in the concentrations of methemoglobin or triiodothyronine were noted between the high-dose and control rats; however, the total thyroxine and the free thyroxine serum levels were decreased in the high-dose rats. Thyroid glands of the high- and mid-dose rats showed moderate follicular cell hyperplasia and misshapen and small follicles. Exposure to 5-amino-o-cresol produced significant hepatotoxic effects manifested by centrilobular hepatocytomegaly in an unspecified number of test rats and one control female.
References
[1]Guo TL, Germolec DR, Zhang LX, Auttachoat W, Smith MJ, White KL Jr. Contact sensitizing potential of pyrogallol and 5-amino-o-cresol in female BALB/c mice. Toxicology. 2013 Dec 15;314(2-3):202-8. doi: 10.1016/j.tox.2013.10.006. Epub 2013 Oct 27. PMID: 24172597; PMCID: PMC3862031.
[2]Eggenreich, K et al. “Determination of 4-amino-m-cresol and 5-amino-o-cresol and metabolites in human keratinocytes (HaCaT) by high-performance liquid chromatography with DAD and MS detection.” Journal of biochemical and biophysical methods vol. 61,1-2 (2004): 23-34. doi:10.1016/j.jbbm.2004.04.021
[3]National Toxicology Program. NTP Technical Report on the Toxicity Studies of 5-Amino-o-cresol (CASRN 2835-95-2) Administered Dermally to F344/NTac Rats and B6C3F1/N Mice: Toxicity Report 89 [Internet]. Research Triangle Park (NC): National Toxicology Program; 2015 Nov.
You may like
See also
Lastest Price from 5-Amino-o-cresol manufacturers

US $1.00/kg2025-06-09
- CAS:
- 2835-95-2
- Min. Order:
- 10kg
- Purity:
- 99 %
- Supply Ability:
- 10000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 2835-95-2
- Min. Order:
- 1KG
- Purity:
- 99%HPLC
- Supply Ability:
- 10 TONS