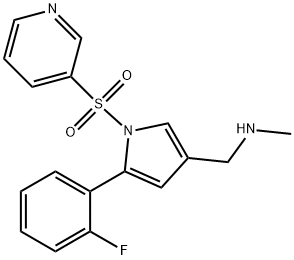
Medical uses and Toxicology of Vonoprazan
Vonoprazan, marketed under the brand name Voquezna among others, is a first-in-class potassium-competitive acid blocker medication. It is used in the form of vonoprazan fumarate to treat gastroduodenal ulcers, including some drug-induced peptic ulcers, and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori. The medication was first approved in Japan in February 2015, later in Russia in April 2021, and received approval for medical use in the United States in November 2023. Co-packaged combinations of vonoprazan with amoxicillin, as well as vonoprazan with both amoxicillin and clarithromycin, are commercially available. [1]
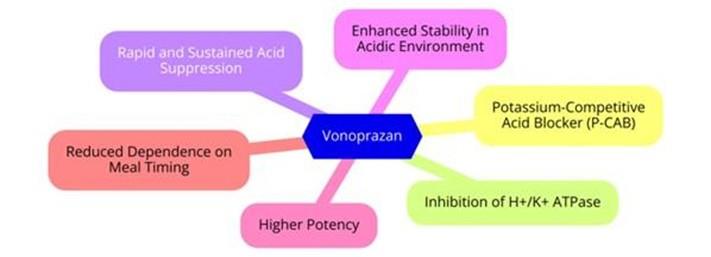
Figure 1: The mechanism of action of vonoprazan
Medical uses
Vonoprazan is indicated for the healing and maintenance of healing of all grades of erosive esophagitis, as well as for relief of heartburn associated with this condition in adults. It is also used in combination with amoxicillin and clarithromycin, or with amoxicillin alone, for the treatment of Helicobacter pylori infection in adults. In July 2024, its indication was expanded in the US to include the treatment of non-erosive gastroesophageal reflux disease.
Drug Interactions
Vonoprazan elevates gastric pH, which may affect the absorption of drugs whose oral bioavailability is highly dependent on gastric acidity. Vonoprazan should not be co-administered with atazanavir or rilpivirine, and caution is advised when Vonoprazan is used with nelfinavir, itraconazole, or tyrosine kinase inhibitors such as gefitinib, nilotinib, and erlotinib, as the efficacy of these drugs may be reduced. Additionally, caution is required when Vonoprazan is taken with digoxin or metildigoxin, since the effects of these drugs may be enhanced. Vonoprazan is primarily metabolized by the hepatic enzyme CYP3A4, with partial metabolism via CYP2B6, CYP2C19, and CYP2D6. Caution is recommended when Vonoprazan is co-administered with the strong CYP3A4 inhibitor clarithromycin, as this may increase Vonoprazan plasma concentrations. In cases of overdose, no specific studies have been conducted, and there are no documented reports of Vonoprazan overdose. Vonoprazan is not removable by hemodialysis. If an overdose occurs, symptomatic and supportive treatment should be provided. [2]
Toxicology
Vonoprazan administered at doses of 30, 100, and 300 mg/kg/day showed no adverse effects on fertility or early embryonic development in male or female rats. Maternal toxicity was observed at doses ≥100 mg/kg/day, including deaths (4/20 males at 300 mg/kg/day), mydriasis, tremors, prone position, soiled fur around the vulva, decreased activity, red nasal discharge, chromodacryorrhea, reduced body weight, and decreased food consumption. The no-observed-adverse-effect level (NOAEL) for maternal toxicity was 30 mg/kg/day, while the NOAEL for reproductive function and early embryonic development was ≥300 mg/kg/day. In rat embryo-fetal development studies, Vonoprazan at doses up to 100 mg/kg/day showed no developmental toxicity. At 300 mg/kg/day, developmental toxicity was observed, including reduced fetal weight, decreased number of caudal vertebrae, and increased incidences of external and visceral abnormalities such as tail malformations, anal stenosis, membranous ventricular septal defects, and abnormal origin of the subclavian artery. Maternal toxicity was noted at doses ≥100 mg/kg/day. In rabbit embryo-fetal development studies, Vonoprazan at doses of 3, 10, and 30 mg/kg/day showed no effects on embryo-fetal development. Maternal toxicity was observed at doses ≥10 mg/kg/day, including reduced feces, decreased body weight or weight gain, and reduced food intake; at 30 mg/kg/day, two females aborted and one dam experienced complete litter loss.
Biological Experiment
In rat perinatal toxicity studies, Vonoprazan at doses up to 10 mg/kg/day showed no significant effects on maternal or F1 offspring development. At 100 mg/kg/day, maternal and fetal toxicity were observed, including reduced pup weight and discoloration of the caudate lobe in the liver on postnatal day 4. No adverse effects on survival, development, or growth were observed in F2 offspring at doses ≤100 mg/kg/day. Vonoprazan and its metabolites are excreted in milk and can cross the placental barrier.
Reference
[1] Echizen H, The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations. Clinical Pharmacokinetics. 2016, 55 : 409–418.
[2] Padwale V. A Comprehensive Review on the Efficacy and Safety of Vonoprazan in the Management of Gastric Acid-Related Diseases. Cureus. 2024, 17 :e64777.
Related articles And Qustion
See also
Lastest Price from Vonoprazan manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 881681-00-1
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 100kg