Uses and Mechanism of Vonoprazan
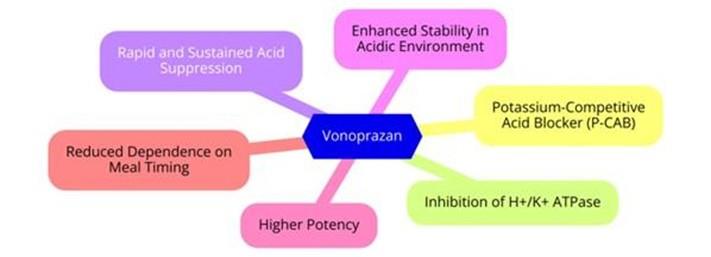
Vonoprazan (trade name Takecab) is a first-in-class potassium-competitive acid blocker. It was approved in the Japanese market in February 2015.

Uses
Vonoprazan is used in form of the fumarate for the treatment of gastroduodenal ulcer (including some drug-induced peptic ulcers) and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori.
Mechanism of action
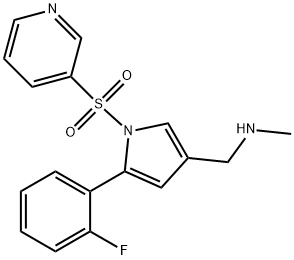
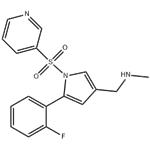
Vonoprazan (TAK-438), a potassium-competitive acid blocker, inhibits acid secretion by competitively blocking availability of potassium to hydrogen-potassium ATPase. Chemically it is a pyrrole derivative (1-[5-(2- fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate) currently being developed by the Takeda Pharmaceutical Company.
Pharmacodynamics
Vonoprazan is acid stable and rapidly absorbed fasting or fed, reaching Cmax by 1.5–2.0 hours. It dissociates slowly from its target (half-life of approximately 7.7 hours). Its high pKa (>9) promotes accumulation in the canalicular space of parietal cells, where it competitively inhibits active and resting proton pumps.2 Interindividual variability in effect exists related to dose, sex, age, and CYP2C19.2 No dosage adjustments are recommended for renal or liver disease.Vonoprazan inhibits CYP2B6 and CYP3A4/5, which extends the metabolism of coadministered drugs such as clarithromycin.
Vonoprazan overcomes many of the perceived weakness of traditional proton pump inhibitor (PPI) therapy (short half-life, destruction in an acid environment requiring acid protection, inhibition of only activated proton pumps, requiring 3–5 cycles of administration before achieving full effect,and clinical variability related to CYP2C19 polymorphisms). 1, 4 Vonoprazan has been approved in Japan for the treatment of gastric and duodenal ulcers, healing of reflux esophagitis and prevention from relapse, secondary prevention of low-dose aspirin or nonsteroidal anti-inflammatory drug–induced gastric mucosal damage, and for first and second-line Helicobacter pylori eradication therapy.
Related articles And Qustion
Lastest Price from Vonoprazan manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 881681-00-1
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 100kg