N-n-Butyl Benzene Sulfonamide: Synthesis and Applications
N-n-Butyl benzene sulfonamide, a colorless and transparent liquid with a characteristic irritating odor under ambient temperature and pressure, is primarily synthesized through an amidation reaction between benzenesulfonyl chloride and n-butylamine in the presence of an appropriate base; and N-n-Butyl benzene sulfonamide, serves mainly as a plasticizer for polymer resins, demonstrating significant applications in both the modification research and industrial production of nylon plastics.

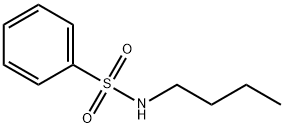
Figure1: Picture of N-n-Butyl benzene sulfonamide
Synthesis
The invention discloses a synthesis method for N-n-Butyl benzene sulfonamide, which primarily utilizes sodium hydroxide, benzenesulfonyl chloride, n-butylamine, sodium metaaluminate, phosphoric acid, dipentaerythritol, ammonium polyphosphate, and tetraethyl orthosilicate as key raw materials; in this innovative process, N-n-Butyl benzene sulfonamide is produced by contacting benzenesulfonyl chloride with an excess of n-butylamine and a basic reagent solution under the action of a porous catalyst, followed by the removal of water and the excess amine from the organic phase, and finally isolating the target product, wherein the application of ultrasonic assistance not only further enhances the reaction yield but also simplifies the subsequent separation and purification procedures, thereby providing a novel approach for the industrial production of the plasticizer N-n-Butyl benzene sulfonamide.
Solid-phase Synthesis
A solvent-free solid-phase synthesis method for N-n-Butyl benzene sulfonamide has been disclosed in a study, comprising the following steps: (1) charging and reaction, where benzenesulfonyl chloride, n-butylamine, and an inorganic alkaline compound are subjected to a solvent-free solid-phase reaction; (2) filtration and washing, where the post-reaction solid is separated and washed with an organic solvent, followed by the combination of the organic phases; and (3) distillation and purification, where the combined organic phases are subjected to vacuum distillation under reduced pressure, collecting the fraction at 150–160 °C/0.5 mmHg to obtain the said N-n-Butyl benzene sulfonamide; this inventive method, which employs benzenesulfonyl chloride, n-butylamine, and an inorganic alkaline compound for the solvent-free solid-phase reaction to synthesize N-n-Butyl benzene sulfonamide, offers advantages such as a simplified process, mild reaction conditions, fast synthesis rate, safety, and environmental friendliness, and the resulting N-n-Butyl benzene sulfonamide possesses high yield, high purity, and excellent quality, endowing it with broad market prospects.
Application
N-n-Butyl benzene sulfonamide is primarily employed as a plasticizer for nylon plastics, polyamides, and cellulose resins to enhance material flexibility, and is also used in latex adhesives, hot-melt adhesives, printing inks, and surface coatings.
Effect on the Mechanical Properties
N-n-Butyl benzene sulfonamide (N-BBSA), as a plasticizer, was investigated for its impact on the mechanical properties and crystallization behavior of Nylon 612, with researchers reporting the effects of varying its addition amounts on the structure and properties of the polymer; the results indicated that N-n-Butyl benzene sulfonamide progressively weakened the intermolecular hydrogen bonding of Nylon 612, leading to a gradual increase in the melt mass-flow rate, elongation at break, and impact strength of the material, while simultaneously causing a decrease in its tensile strength, flexural strength, and flexural modulus, and furthermore, a higher addition content resulted in lower crystallization and melting temperatures, with the material's degree of crystallinity first decreasing and then increasing. [2]
Effects of plasticizers
Researchers prepared CIIR/polyamide 12 (PA12) thermoplastic vulcanizates (TPVs) via dynamic vulcanization and investigated the effects of plasticizers—naphthenic oil, polyisobutylene, and N-n-Butyl benzene sulfonamide—on the physical, rheological, crystallization, dynamic mechanical properties, and air-tightness of the CIIR/PA12 TPVs, with the results demonstrating that N-n-Butyl benzene sulfonamide exhibited a certain plasticizing effect; specifically, when compared to naphthenic oil and polyisobutylene and at a lower dosage, it significantly reduced the TPV hardness and improved the extrudate appearance and processability while largely maintaining the physical properties and air-tightness, and furthermore, upon its incorporation, the glass transition temperature (Tg) of the PA12 phase was reduced by up to approximately 40°C without adversely affecting the crystallinity of the plastic phase.
Reference
[1] Sun, Q., Wu, J. G., & Wan, H. B. A synthesis method of N-butyl benzenesulfonamide: CN201811092745.X[P].
[2] Sun, G. W., & Zuo, H. T. Influence of N-Butyl Benzenesulfonamide on the Mechanical Properties and Crystallization Behavior of Nylon 612. Shanghai Plastics, 2025(2).
See also
Lastest Price from N-n-Butyl benzene sulfonamide manufacturers

US $0.00/kg2025-08-29
- CAS:
- 3622-84-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $4.00/kg2025-04-21
- CAS:
- 3622-84-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000