Application research of 4-pyridinecarboxaldehyde
Introduction
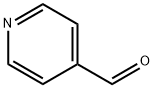
Substituted pyridines have attracted much attention due to their multiple applications. 4-Pyridinecarboxaldehyde (4PCA; Fig. 1) was shown to be an efficient building block for synthesis of Schiff bases using a Korich-type reaction, an intermolecular reductive synthetic procedure based on the condensation of nitro-substituted arenes and aromatic aldehydes in the presence of iron powder and dilute acid. Metal complexes of several of these Schiff bases were shown to exhibit good activity against different types of bacteria (e.g., Staphylococcus aureus, E. coli, Klebsiella, Pneumonia) and fungi (Candida albicans, Apergillusniger and Pencillium sp), and also moderate nuclease activity. 4-Pyridinecarboxaldehyde and some of its derivatives have also been reported as useful transamination reagents to introduce ketone or aldehyde groups onto the N-termini of antibodies for subsequent site-specifically conjugate aminooxy-functionalized molecules (including fluorescent dyes,polyethylene glycol, or porphyrins) to these entities.[1]

Matrix isolation infrared spectroscopic study of 4-Pyridinecarboxaldehyde and of its UV-induced photochemistry
In the present study, (i) the equilibrium geometry of 4PCA and the barrier of internal rotation of the aldehyde group were obtained at a higher-level of theory than previously reported (Moller-Plesset to second order with a triple-ζ basis set, completed with polarization functions in both heavy atoms and hydrogens as well as with diffuse functions: MP2/6-311++G(d,p)), (ii) the infrared spectrum of the compound isolated in an argon matrix was studied in an extended frequency range (4000–400 cm−1 ) and fully assigned based on normal coordinates' calculations, and (iii) the UV-induced photochemistry exhibited by the matrix-isolated compound was investigated. The molecule has a planar structure (Cs point group), with MP2/6-311++G(d,p) predicted internal rotation barrier of 26.6kJmol-1, which is slightly smaller than that of benzaldehyde (~30kJmol-1), thus indicating a less important electron charge delocalization from the aromatic ring to the aldehyde moiety in 4PCA than in benzaldehyde. A complete assignment of the infrared spectrum of 4PCA isolated in an argon matrix has been done for the whole 4000-400cm-1 spectral range, improving over previously reported data. Both the geometric parameters and vibrational frequencies of the aldehyde group reveal the relevance in this molecule of the electronic charge back-donation effect from the oxygen trans lone electron pair to the aldehyde CH anti-bonding orbital. Upon in situ UV irradiation of the matrix-isolated compound, prompt decarbonylation was observed, leading to formation of pyridine.[1]
4-Pyridinealdazine as a Bridging Ligand
The ligand 4-pyridinealdazine has been used for connecting metallic centers in polymeric structures; a good electronic communication is expected, considering the electron delocalization promoted by including free N atomic orbitals in the bridge. Novel polynuclear complexes of rhenium and ruthenium containing PCA (PCA=4-pyridinecarboxaldehyde azine or 4-pyridinealdazine or 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene) as a bridging ligand have been synthesized as PF6- salts and characterized by spectroscopic, electrochemical, and photophysical techniques. The precursor mononuclear complex, of formula [Re(Me2bpy)(CO)3(PCA)]+ (Me2bpy = 4,4'-dimethyl-2,2'-bipyridine), does not emit at room temperature in CH3CN, and the transient spectrum found by flash photolysis at lambda(exc)=355nm can be assigned to a MLCT (metal-to-ligand charge transfer) excited state [(Me2bpy)(CO)3ReII(PCA-)]+, with lambda(max) = 460nm and tau < 10 ns. The spectral properties of the related complexes [[Re(Me2bpy)(CO)3}2(PCA)]2+, [Re(CO)3(PCA)2Cl], and [Re(CO)3Cl]3(PCA)4 confirm the existence of this low-energy MLCT state. The dinuclear complex, of formula [(Me2bpy)(CO)3Re(I)(PCA)Ru(II)(NH3)5]3+, presents an intense absorption in the visible spectrum that can be assigned to a MLCT d(pi)(Ru) --> pi(PCA); in CH3CN, the value of lambda (max) = 560 nm is intermediate between those determined for [Ru(NH3)5(PCA)]2+ (lambda(max)=536nm) and [(NH3)5Ru(PCA)Ru(NH3)5]4+ (lambda(max) = 574nm), indicating a significant decrease in the energy of the pi-orbital of PCA. The mixed-valent species, of formula [(Me2bpy)(CO)3Re(I)(PCA)Ru(III)(NH3)5]4+, was obtained in CH3CN solution, by bromine oxidation or by controlled-potential electrolysis at 0.8 V in a OTTLE cell of the [Re(I),Ru(II)] precursor; the band at lambda(max) = 560 nm disappears completely, and a new band appears at lambda(max) = 483 nm, assignable to a MMCT band (metal-to-metal charge transfer) Re(I) --> Ru(III). By using the Marcus-Hush formalism, both the electronic coupling (HAB) and the reorganization energy (lambda) for the metal-to-metal intramolecular electron transfer have been calculated. Despite the considerable distance between both metal centers (approximately 15.0 Angstroms), there is a moderate coupling that, together with the comproportionation constant of the mixed-valent species [(NH3)5Ru(PCA)Ru(NH3)5]5+ (K(c) approximately 102, in CH3CN), puts into evidence an unusual enhancement of the metal-metal coupling in the bridged PCA complexes. This effect can be accounted for by the large extent of "metal-ligand interface", as shown by DFT calculations on free PCA. Moreover, lambda is lower than the driving force -DeltaG degrees for the recombination charge reaction [Re(II),Ru(II)]-->[Re(I),Ru(III)] that follows light excitation of the mixed-valent species. It is then predicted that this reverse reaction falls in the Marcus inverted region, making the heterodinuclear [Re(I),Ru(III)] complex a promising model for controlling the efficiency of charge-separation processes.[2]
Emission and excitation spectra of 4-pyridinecarboxaldehyde
Emission and excitation spectra of 2-, 3- and 4-pyridinecarboxaldehyde (2-, 3- and 4-PCA, respectively) vapors have been measured at different temperatures and compared to one another. The emission spectra of these vapors are shown to consist of the T1(n, π*) --> S(0) phosphorescence accompanied by the weak thermally activated S1(n, π*) --> S0 delayed fluorescence. Two peaks originating from the two rotamers (syn and anti) have been identified in the fluorescence, phosphorescence and excitation spectra of 3-pyridinecarboxaldehyde vapor. Analyses of the temperature dependence and vibrational structure of the spectra of 3-pyridinecarboxaldehyde vapor provide the syn-anti energy difference of 190±30 cm-1 in the T(1) (n, pi) state, 200±30 cm-1 in the S(1)(n, pi) state, and 290±35 cm-1 in the ground state. The ground-state energy difference is in agreement with the result of density functional theory (DFT) calculation for 3-pyridinecarboxaldehyde vapor. DFT calculation demonstrated also that the syn rotamer exists as a less stable isomer in the ground state for 2- and 3-pyridinecarboxaldehyde vapors.[3]
References
1. Cluyts L, Sharma A, Kuş N, Schoone K, Fausto R. Matrix isolation infrared spectroscopic study of 4-Pyridinecarboxaldehyde and of its UV-induced photochemistry. Spectrochim Acta A Mol Biomol Spectrosc. 2017;171:207-212. doi:10.1016/j.saa.2016.08.002
2. Cattaneo M, Fagalde F, Katz NE, Leiva AM, Schmehl R. Enhancement of metal-metal coupling at a considerable distance by using 4-pyridinealdazine as a bridging ligand in polynuclear complexes of rhenium and ruthenium. Inorg Chem. 2006;45(1):127-136. doi:10.1021/ic051312b
3.Itoh T. Emission spectra and electronic energy levels of the rotational isomers of pyridinecarboxaldehyde vapors. J Phys Chem A. 2006;110(33):10012-10017. doi:10.1021/jp068043a
You may like
Related articles And Qustion
Lastest Price from 4-Pyridinecarboxaldehyde manufacturers

US $10.00/KG2025-04-21
- CAS:
- 872-85-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-04-04
- CAS:
- 872-85-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton