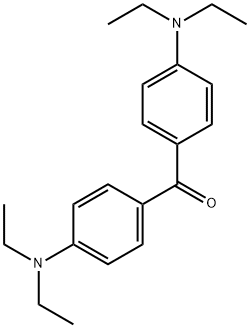
4,4’-Bis(diethylamino)benzophenone: application,embryotoxicity and a determination method
Introduction
Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI), as an emerging label-free molecular imaging approach, has developed as a promising analytical technique capable of generating spatially resolved imaging of various endogenous compounds (for example, metabolites, nucleotides, peptides, proteins, and lipids) in tissue specimens. Thus, MALDI-MSI has been widely applied in diverse fields of zoology, basic medical science,pharmaceutical science, plant science, environmental science, and forensic science. 4,4'-Bis(diethylamino)benzophenone, also named as Michler's ethylketone (Figure 1), has been successfully screened and optimized as a new MALDI matrix for the enhancement of tissue imaging of lipids directly from rat liver,rat brain and germinating Chinese-yew (Taxus chinensis var.mairei) seed tissues by MALDI time-of-fight (TOF)/TOF MS in negative-ion mode.Many studies show several properties of 4,4’-bis(diethylamino)benzophenone as a powerful MALDI matrix, including strong UV absorption,lm-sized crystals and uniform matrix-coating, super high vacuum chemical stability, low matrix-related ion interference, super soft ionization, and high lipid ionization efficiency.[1]

4,4'-Bis(diethylamino)benzophenone used as glucose sensor
a new low dimensional donor–acceptor (D–A) material from 4,4'-bis(diethylamino)benzophenone (BZP) in which two donor diethyl phenyl amine units are bridged by the accepter keto group have been introduced (Scheme 1). Aggregated 4,4’-bis(diethylamino)benzophenone molecule exhibits exceptional blue shifted PL spectra with a strong intense emission in its aggregated state than its dilute solution in any good solvent. This aggregation induced enhanced (AIE) blue emission is explained due to suppression of twisted intramolecular charge transfer (TICT) state in the aggregated structure and the blue shifted emission appears from the locally excited (LE) state of 4,4'-bis(diethylamino)benzophenone in its aggregated structures. A broad red shifted emission band was also observed and it increased inintensity with increasing size of the aggregates. This broad emission originated from the excited intramolecular charge transfer (ICT) state of the relatively planar molecule present within the aggregated structures of 4,4'-bis(diethylamino)benzophenone due to crystal softening.Mazumdar etal. have also used this aggregated material in fluorescence sensory probe and found its quenching efficiency in the presence of D-glucose. Quenching of blue emission from the aggregated structure has been explained due to the hydrogen bonding complexation between D-glucose and BZP molecule present in the aggregated structures.[2]
The embryotoxicity of 4,4'-bis(diethylamino)-benzophenone in stem cells and zebrafish embryos
Benzophenones (BPs) are widely used as photoinitiators (PIs) or printing inks in food packaging, which may migrate into foods. However, the toxicity information of some BP analogues, such as 4,4'-bis(diethylamino)-benzophenone (DEAB), 4-phenylbenzophenone (4-PBP), 4 (hydroxymethyl)benzophenone (4-HMBP), those are used as PIs is lacking. Developmental toxicity is a health concern associated with PIs exposure. Recently, alternative non-in vivo methods have been proposed to evaluate the concerned chemicals or better understand the modes of action of certain toxicological endpoints. In this study, using in silico methods, we predicted that BP, DEAB, 4-PBP and 4-HMBP might exhibit developmental toxicity. However, we found that only 4,4'-bis(diethylamino)-benzophenone is strong embryotoxic and disturbs the early differentiation of mouse embryonic stem cells into three germ layers and cardiomyocytes. 4,4'-Bis(diethylamino)-benzophenone treatment also prevented cardiomyocyte differentiation in human induced pluripotent stem cells (hiPSCs) on day 10. However, BP, 4-PBP and 4-HMBP had no similar effects on cardiomyocyte differentiation on day 10. Transcriptomic analysis revealed that treatment with DEAB significantly decreased the mRNA levels of differentiation-related transcription factors SOX17 and FOXA1, in hiPSCs on day 4. Furthermore, 4,4'-bis(diethylamino)-benzophenone treatment caused tail malformations and yolk sac edema in zebrafish embryos. To conclude, 4,4'-bis(diethylamino)-benzophenone may be embryotoxic because it disturbs the early differentiation of stem cells. Further studies are warranted to better understand the health effects of 4,4'-bis(diethylamino)-benzophenone exposure.[3]
Evaluation of the migration of 15 photo-initiators by UPLC-MS/MS method
Photo-initiators are widely used to cure ink on packaging materials used in food applications such as plastic films or cartonboards. In migration studies, food simulants are very often used to simulate food, like Tenax®, which is the simulant for dry foodstuffs. In this paper a fast and reliable confirmation method for the determination of the following photo-initiators in Tenax® is described: benzophenone (BP), 4,4'-bis(diethylamino)benzophenone (DEAB), 2-chloro-9H-thioxanthen-9-one (CTX), 1-chloro-4-propoxy-9H-thioxanthen-9-one (CPTX), 2,4-diethyl-9H-thioxanthen-9-one (DETX), 2,2-dimethoxy-2-phenyl acetophenone (DMPA), 4-(dimethylamino)benzophenone (DMBP), 2-ethylanthraquinone (EA), ethyl-4-dimethylaminobenzoate (EDMAB), 1-hydroxylcyclohexyl phenyl ketone (HCPK), 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone (HMMP), 2-isopropyl-9H-thioxanthen-9-one (ITX), 4-methylbenzophenone (MBP), Michler's ketone (MK), and 4-phenylbenzophenone (PBZ). After the migration study was completed, the simulant Tenax(®) was extracted using acetonitrile, followed by analysis on ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Quantification was carried out using benzophenone-d10 (BP-d10) as internal standard. The presented method is validated in terms of matrix effect, specificity, linearity, recovery, precision and sensitivity, showing the method can detect all photo-initiators at very low concentrations (LOD<0.125 µg/g for all substances). Finally, the procedure was applied to real samples, proving the capabilities of the presented method.[4]
References
1.Shi Y, Hu H, Hao Q, et al. Michler's ethylketone as a novel negative-ion matrix for the enhancement of lipid MALDI tissue imaging. Chem Commun (Camb). 2022;58(5):633-636. Published 2022 Jan 13. doi:10.1039/d1cc05718a
2.Mazumdar P, Das D, Sahoo GP, Salgado-Morán G, Misra A. Aggregation induced emission enhancement of 4,4'-bis(diethylamino)benzophenone with an exceptionally large blue shift and its potential use as glucose sensor. Phys Chem Chem Phys. 2015;17(5):3343-3354. doi:10.1039/c4cp03772c
3.Weng CY, Chang TC, Liou JY, et al. Evaluating the embryotoxicity of benzophenone-based photoinitiators in stem cells and zebrafish embryos. Toxicology. 2024;508:153930. doi:10.1016/j.tox.2024.153930
4.Van Den Houwe K, van de Velde S, Evrard C, Van Hoeck E, Van Loco J, Bolle F. Evaluation of the migration of 15 photo-initiators from cardboard packaging into Tenax(®) using ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2014;31(4):767-775. doi:10.1080/19440049.2014.886340
You may like
Lastest Price from 4,4'-Bis(diethylamino) benzophenone manufacturers
US $0.00/kg2025-09-02
- CAS:
- 90-93-7
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $1.00/kg2025-04-21
- CAS:
- 90-93-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10mt