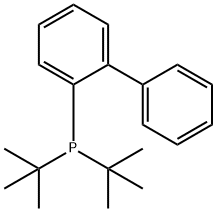
Main application research of 2-(Di-tert-butylphosphino)biphenyl
Introduction
2-(Di-tert-butylphosphino)biphenyl(JohnPhos;Figure.1) is an emblematic Buchwald phosphine, whose steric demands exclude the possibility that gold atoms in neighbouring molecules exhibit any aurophilic contacts and therefore these centres establish other kinds of weak interactions. For example, Au-πPhenyl contacts are stronger and more recurrent than one could expect in this series of compounds. This interaction along with incipient anagostic Au-H interactions and Au-F contacts could indeed influence the chemical bonding between the 2-(Di-tert-butylphosphino)biphenyl ligand and gold(I) centres.[1] With the realization that the reaction required a bulky,electron-rich phosphine, 2-(di-tert-butylphosphino)biphenyl was chosen as the ligand of choice. This decision was basedon the ease of handling of this stable, white solid in contrast to tri-tert-butylphosphine, which oxidizes quickly when exposed to air. This report will introduce its main application research.

Influence of biaryl phosphine structure on C-N and C-C bond formation
In order to understand how electronic and other structural characteristics of biphenyl phosphine ligands affect Pd-catalyzed C-N and C-C bond-forming reactions, a new ligand, 2-(dicyclohexylphosphino)-4'-(N,N-dimethylamino)-1,1'-biphenyl, was synthesized. This compound is isomeric with the commercially available 2-(dicyclohexylphosphino)-2'-(N,N-dimethylamino)-1,1'-biphenyl that has been useful in C-N bond-forming reactions of nucleosides. The new p-dimethylamino biphenyl ligand bears electronic similarities to the o-dimethylamino isomer, but it also possesses structural similarities to 2-(dicyclohexylphosphino)biphenyl, such as the unsubstituted ortho positions in the non-phosphine ring. Whereas 2-(dicyclohexylphosphino)biphenyl can support catalysts for C-C bond formation, it was not effective in promoting aryl amination of a nucleoside substrate. However, the new ligand proved to be effective in promoting both aryl amination and C-C bond-forming reactions of nucleoside substrates, with some reactions even occurring at room temperature. Thus, the composite structural elements of this new ligand are thought to be criteria for reactivity of the catalytic system derived from it. We have probed the structures of the isomeric N,N-dimethylamino biphenyl ligands by X-ray crystallographic analysis. Interactions of the two ligands with Pd(OAc)2 have been investigated by (31)P NMR, and they show substantial stoichiometry-dependent differences. These results have been compared to the interactions of Pd(OAc)2 with 2-(dicyclohexylphosphino)biphenyl as well as 2-(di-tert-butylphosphino)biphenyl, and they reveal marked differences as well. In this process, three cyclopalladated biaryl derivatives have been isolated and characterized by X-ray analysis.[2]
2-(Di-tert-butylphosphino)biphenyl as a ligand involved in the reaction
Reporte 1: In this study, Hennessy and his colleages had reported on the development of a novel variant of the Friedel-Crafts procedure using palladium-catalyzed C-H functionalization, obviating the need for harsh reaction conditions. Specifically, the combination of catalytic amounts of palladium acetate, 2-(di-tert-butylphosphino)biphenyl (1) as a ligand, and triethylamine as base, α-chloroacetanilides can be smoothly converted to oxindoles in high yields with high levels of regioselectivity. The requisite substrates are easily prepared by the condensation of the corresponding aniline with the inexpensive chloroacetyl chloride. Among the phosphine ligands examined,only 2-(di-tert-butylphosphino)biphenyl is highly effective for this transformation. Even structurally similar phosphines commonly used in cross-coupling reactions provided considerably lower amounts of oxindole in the time reactions employing 2-(di-tert-butylphosphino)biphenyl proceeded to nearly complete conversion. This is a reliable and operationally simple protocol to regioselectively synthesize substituted oxindoles.The high levels of functional group tolerance without the need for ortho-halogenation should make this an attractive synthetic alternative to currently used methods.[3]
Report 2: Kakizoe and his colleages had reported the synthesis and photophysical properties of Cu(i) complexes with 1,10-phenanthroline (phen) and monodentate phosphine ligands. Single crystal X-ray structural analysis revealed that these have three-coordinated trigonal planar geometries. They also found that one of them, [Cu(phen)(Johnphos)]BF4 (Johnphos = 2-(di-tert-butylphosphino)biphenyl), is considerably emissive both in solution and solid states. The emission maximum wavelength of the emission of the complex is 580 nm, and the lifetime of the emission is 2 μs in solution. Moreover, we have systematically investigated the photophysical and redox properties of four-coordinate complexes [Cu(NN)(P)2]+ in addition to three-coordinate complexes [Cu(NN)(P)]+. Charge transfer transitions play a key role in the photophysics of these complexes.[4]
Report 3: During our efforts toward the synthesis of naturally occurring polyprenylated polycyclic acylphloroglucinol using a Au(I)-catalyzed 6-endo dig carbocyclization, McGee and his colleages isolated stable vinyllic gold intermediates. Optimization lead to isolated yields of up to 98%, using 2-(di-tert-butylphosphino)biphenyl as the ligand. This transformation is derived from a silyl rearrangement that can be fully controlled according to the nature of the substituent on the ynone. This selective transformation does not require basic conditions to prevent protodeauration. These vinylgold complexes are the first isolated intermediates during a silyl migration with gold(I). More than 16 new organogold complexes were synthesized and characterized by single-crystal X-ray diffraction. Reactivity of these complexes is also presented.[5]
References
1. Moreno-Alcántar G, Hess K, Guevara-Vela JM, et al. π-Backbonding and non-covalent interactions in the JohnPhos and polyfluorothiolate complexes of gold(i). Dalton Trans. 2017;46(37):12456-12465. doi:10.1039/c7dt00961e
2. Pratap R, Parrish D, Gunda P, Venkataraman D, Lakshman MK. Influence of biaryl phosphine structure on C-N and C-C bond formation. J Am Chem Soc. 2009;131(34):12240-12249. doi:10.1021/ja902679b
3.Hennessy EJ, Buchwald SL. Synthesis of substituted oxindoles from alpha-chloroacetanilides via palladium-catalyzed C[bond]H functionalization. J Am Chem Soc. 2003;125(40):12084-12085. doi:10.1021/ja037546g
4.Kakizoe D, Nishikawa M, Fujii Y, Tsubomura T. Photophysical properties of three coordinated copper(i) complexes bearing 1,10-phenanthroline and a monodentate phosphine ligand. Dalton Trans. 2017;46(43):14804-14811. doi:10.1039/c7dt02938a
5.McGee P, Bellavance G, Korobkov I, Tarasewicz A, Barriault L. Synthesis and Isolation of Organogold Complexes through a Controlled 1,2-Silyl Migration. Chemistry. 2015;21(27):9662-9665. doi:10.1002/chem.201501648
You may like
Related articles And Qustion
Lastest Price from 2-(Di-tert-butylphosphino)biphenyl manufacturers

US $0.00-0.00/KG2025-04-21
- CAS:
- 224311-51-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt
US $0.00/g2025-04-12
- CAS:
- 224311-51-7
- Min. Order:
- 1g
- Purity:
- 99%;sales036@researchvip.com;18903931628;
- Supply Ability:
- 100KG