Application research of 2,6-Dibromopyridine
Introduction
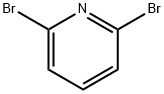
2,6-Dibromopyridine(Figure 1) is a conventional organic chemical product. It is insoluble in water but soluble in solvents such as methanol, ethanol, dioxane, and chloroform. It is primarily utilized in the production of high-end fine chemicals, including pharmaceuticals and pesticides. Ethyl 2,6-pyridinedicarboxylate can be synthesized from 2,6-dibromopyridine and is employed in the development of engineering plastics, as well as novel pesticides and pharmaceutical products. This paper mainly introduces its application research.

Application research examples
1.Synthesis of Diaminated Proligands
Selective mono- or diaminations of 2,6-dibromopyridine were performed using microwave irradiation with water as solvent in 2-2.5 h. The only significant difference between the syntheses was the inclusion of K2CO3 as base and CuI/DMPAO catalyst for the diaminations. The mono- and diaminations had approximately 7 and 2 g isolated yields, respectively. The monoaminated bromopyridines were attached to a TREN scaffolding molecule yielding novel ligands to support extended metal atom chain complexes. There have also been studies investigating catalyzed asymmetric aminations of 2,6-dibromopyridine with primary and secondary amines, but these also rely on long reaction times (12-48h) and conventional heating. The approach used herein to promote C−N bond formation was to use a commercially available Cu/L catalyst in combination with a microwave synthesizer under relatively mild conditions. There are examples of uncatalyzed and Cu catalyzed, microwave assisted C−C and C−heteroatom bond forming reactions as well as reactions that operate in milder conditions, for example with aqueous ammonia and Cu2O as a catalyst employing conventional heating or microwave irradiation, but these studies do not provide a generalized approach.[1]
2.Acylation of Alkenes
Friedel-Crafts-type acylation of alkenes with acyl chlorides has been successfully conducted with a wide substrate scope by the combined use of AlCl3 and 2,6-dibromopyridine. Trisubstituted alkenes afford allylketones or vinylketones depending on the presence or absence of hydrogen atom(s) at the β-position to the acylation site, while monosubstituted alkenes exclusively afford vinylketones.Notably, various alkenes were acylated in the presence of 2,6-dibromopyridine without undergoing polymerization.Terminal dialkylalkenes, which easily undergo isomerization,could be acylated into enones corresponding to the original alkenes by the addition of 2,6-di-tert-butylpyridine or bythe use of EtAlCl2 with 2,6-dibromopyridine.[2]
3.Carboxylation reaction of Alkenes with Carbon Dioxide
α-Arylalkenes and trialkyl-substituted alkenes undergo carboxylation with CO2 in the presence of EtAlCl2 and 2,6-dibromopyridine to afford the corresponding α,β- and/or β,γ-unsaturated carboxylic acids. This reaction is suggested to proceed via the electrophilic substitution of EtAlCl2 with the aid of the base, followed by the carbonation of the resulting ate complex. This reaction can be applied to terminal dialkylalkenes by using a mixture of 2,6-di-tert-butylpyridine and 2,6-dibromopyridine.[3]
4.Suzuki-Miyaura coupling reaction
Report 1: Expanded porphyrins have received considerable attention due to their unique optical, electrochemical and coordination properties. Here, researchers report benzene- and pyridine-incorporated octaphyrins(1.1.0.0.1.1.0.0), which are synthesized through Suzuki-Miyaura coupling of α,α'-diboryltripyrrane with m-dibromobenzene and 2,6-dibromopyridine, respectively, and subsequent oxidation with 2,3-dicyano-5,6-dichlorobenzoquinone. Both octaphyrins are nonaromatic and take on dumbbell structures. Upon treatment with Pd(OOCCH3)2, the benzene-incorporated one gives a Ci symmetric NNNC coordinated bis-PdII complex but the pyridine incorporated one gives Ci and Cs symmetric NNNC coordinated bis-PdII complexes along with an NNNN coordinated bis-PdII complex bearing a transannular C-C bond between the pyrrole α-positions. In addition, these two pyridine-containing NNNC PdII complexes undergo trifluoroacetic acid-induced clean interconversion.[4]
Report 2: m-Pyripentaphyrins(1.0.0.0.0) were synthesized by Suzuki-Miyaura coupling of 3,5-bis(5-borylpyrrol-2-yl)-BODIPY with 2,6-dibromopyridine. Upon treatment with PhBCl2, pyripentaphyrin 1 provided mono- and bis-BIII complexes sequentially. The Mono-BIII complex shows a distorted tetrahedral coordinated BIII with a σ-phenyl ligand on the BIII and the bis-BIII complex shows an additional distorted tetrahedral coordinated BIII with a B-H bond. Bromination of the pyripentaphyrins with N-bromosuccinimide (NBS) resulted in regioselective formation of 8-bromopyripentaphyrins, which were dimerized to 8,8'-linked dimers by reductive coupling with Ni(cod)2. While all these pyripentaphyrins are nonaromatic, they exhibit characteristic broad absorption bands at long wavelength near the NIR region, indicating the presence of effective macrocyclic conjugation.[5]
References
1. Underwood AS, Botrous MA, Lobb NT, et al. Selective Mono- and Diamination of 2,6-Dibromopyridine for the Synthesis of Diaminated Proligands. ACS Omega. 2025;10(32):36321-36327. Published 2025 Aug 11. doi:10.1021/acsomega.5c04396
2. Tanaka S, Kunisawa T, Yoshii Y, Hattori T. Acylation of Alkenes with the Aid of AlCl3 and 2,6-Dibromopyridine. Org Lett. 2019;21(21):8509-8513. doi:10.1021/acs.orglett.9b02688
3. Tanaka S, Watanabe K, Tanaka Y, Hattori T. EtAlCl2/2,6-Disubstituted Pyridine-Mediated Carboxylation of Alkenes with Carbon Dioxide. Org Lett. 2016;18(11):2576-2579. doi:10.1021/acs.orglett.6b00918
4. Liu L, Hu Z, Zhang F, et al. Benzene- and pyridine-incorporated octaphyrins with different coordination modes toward two PdII centers. Nat Commun. 2020;11(1):6206. Published 2020 Dec 4. doi:10.1038/s41467-020-20072-9
5. Wu L, Li P, Xu L, et al. m-Pyripentaphyrins(1.0.0.0.0): BIII Complexes and β-β Directly Linked Dimers. Chem Asian J. 2024;19(19):e202400649. doi:10.1002/asia.202400649You may like
See also
Lastest Price from 2,6-Dibromopyridine manufacturers

US $19.90/kg2025-04-21
- CAS:
- 626-05-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $15.00-50.00/kg2025-04-02
- CAS:
- 626-05-1
- Min. Order:
- 1kg
- Purity:
- NLT98%
- Supply Ability:
- 5 ton per month