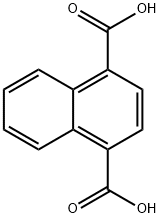
Synthesis and Coordination Properties of 1,4-Naphthalenedicarboxylic Acid
1,4-Naphthalenedicarboxylic acid, an aromatic carboxylic acid compound, exhibits remarkable chemical stability, distinct fluorescent properties, and significant acidity. While insoluble in pure water, this compound demonstrates measurable solubility in alkaline aqueous solutions. As a pivotal organic chemical raw material, 1,4-naphthalenedicarboxylic acid enables the synthesis of diverse industrial products: through polycondensation with diols such as ethylene glycol, it yields polyethylene naphthalate (PEN) and other polyester polymers essential for manufacturing high-performance plastics and synthetic fibers. Furthermore, this versatile acid serves as a key intermediate in pigment production, contributing to the synthesis of azo dyes and anthraquinone dyes while enhancing critical performance parameters including color fastness and chromatic stability.
Synthesis
Method 1
The invention discloses a preparation method for 1,4-naphthalenedicarboxylic acid, which comprises the following steps: dissolving 1-methyl-4-naphthoic acid in glacial acetic acid, adding cobalt acetate, manganese acetate and sodium acetate in a mass ratio of 1:50 to the mixture, heating to 90°C until complete dissolution, then introducing a catalyst, raising the temperature to 130°C with uniform air injection, followed by cooling and filtration to afford the crude product; acetic anhydride and supplementary catalyst are added to the mother liquor for further reaction with 1-methyl-4-naphthoic acid; the crude product is dissolved in water, mixed with caustic soda flakes, heated to 70–90°C, adjusted to pH 7–8, treated with activated carbon under stirring and suction filtration, reheated to 70–90°C, acidified to pH below 2, cooled to 20–30°C for centrifugal separation, and the solid is washed to neutrality and dried to give the final product 1,4-Naphthalenedicarboxylic acid.
Method 2
1,4-Naphthalenedicarboxylic acid was synthesized from 1,4-dimethylnaphthalene by high performance liquid phase catalytic oxidation. The effects of different reaction conditions on the yield and regioselectivity,such as substrate concentration,temperature,reaction time and so on,were also investigated. The optimal reaction conditions were determined by studying the above factors.
Method 3
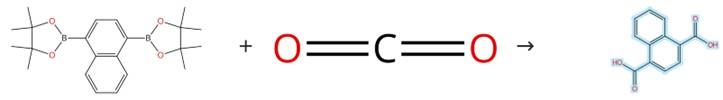
Figure 1: Synthesis of 1,4-Naphthalenedicarboxylic Acid
A 30 mL stainless steel autoclave containing organoboronic acid pinacol ester (0.5 mmol) and (IPr)CuCl (0.025 mmol, 5 mol%) is dried under vacuum and transferred into a nitrogen-atmosphere glovebox, where tBuOK (0.025 mmol, 5 mol%), CsF (1.00 mmol, which has been pre-dried at 80°C for 12 h under vacuum and cooled to room temperature), and anhydrous 1,4-dioxane (1.0 mL) are added; after sealing, the autoclave is removed from the glovebox, pressurized with CO2 to 2.0 MPa, and stirred at 160°C for 24 h, then cooled to room temperature with slow release of residual CO2; the reaction mixture is acidified with 1 M HCl(aq), extracted twice with EtOAc, and the combined organic layers are washed with brine, dried over Na2SO4, concentrated under reduced pressure, and finally purified by silica gel column chromatography to afford the product 1,4-Naphthalenedicarboxylic acid . [1]
Coordination Properties
On moving to the organic linker point of view, multidentate carboxylate functionalities are considered to be better candidates, because they are known to chelate and lock metal ions in clusters, which act as rigid entities. In this regard, as the analog of the most familiar linear terephthalate rigid linker, 1,4-naphthalenedicarboxylic acid (H2NDA) has been confirmed to act as a good candidate in the preparation of novel CPs with interesting properties. And also, to the best of our knowledge, no attempts were made to synthesize s-block coordination poly mers based on 1,4-naphthalenedicarboxylic acid ligand.
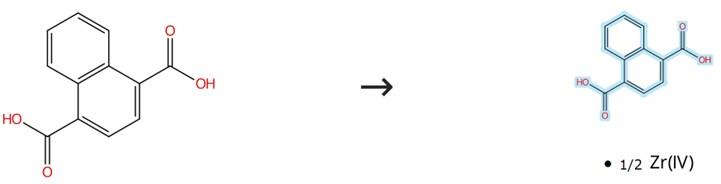
Figure 2: Coordination Properties of 1,4-Naphthalenedicarboxylic Acid
To a homogenous mixture of 4 mL DMF under continuous stirring, zirconium tetrachloride (0.233 g, 0.1 mmol), 1,4-Naphthalenedicarboxylic acid (0.432 g, 0.2 mmol), and glacial acetic acid (2 mL) are sequentially introduced and maintained under agitation for 15 minutes until achieving complete dissolution of all components, after which the resulting clear solution is carefully transferred into a 25 mL Teflon-lined stainless steel autoclave for hydrothermal treatment at 100°C over 24 hours; following gradual cooling to ambient temperature, the crystalline product is efficiently separated from the mother liquor via centrifugation and subjected to multiple washing cycles with fresh DMF and methanol to remove residual solvents and unreacted species, ultimately yielding the purified material after overnight drying at room temperature under atmospheric conditions.[2]
Reference
[1] Maeda, Chihiro; et al, Cu-catalyzed carboxylation of organoboronic acid pinacol esters with CO2, Organic & Biomolecular Chemistry 2023, 21, 6565-6571.
[2] Zheng, Jin-Yang; et al, Enhanced proton conductivity by incorporating sulfonic acid groups into a zirconium-based metal-organic framework via ligand exchange, Journal of Solid State Chemistry 2023, 324, 124070.
See also
Lastest Price from 1,4-Naphthalenedicarboxylic acid manufacturers

US $0.00/kg2025-08-22
- CAS:
- 605-70-9
- Min. Order:
- 1kg
- Purity:
- 99%Min
- Supply Ability:
- 20tons

US $1000.00/KG2025-08-13
- CAS:
- 605-70-9
- Min. Order:
- 1KG
- Purity:
- 98%HPLC
- Supply Ability:
- 200tons