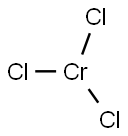
Synthesis and Toxicological Profile of Chromium(III) Chloride Anhydrous
Introduction
CrCl3 is not ionic, but rather has a network solid structure with bridged Cl- ions and octahedrally coordinated Cr (III) ions, which form layers. Although chromium(III) chloride hexahydrate is green, solid chromium chloride anhydrous is purple. The solid consists of shiny flakes, owing to its layered structure. The stability of the network structure makes CrCl3 insoluble in water and most other solvents. [1]

The Synthesis of Chromium Chloride Anhydrous
The reaction is carried out at 700 °C in a Vycor (quartz) tube in a tube furnace. Nitrogen is bubbled through liquid carbon tetrachloride, and the stream of nitrogen / carbon tetrachloride is passed over a pile of green chromium(III) oxide in the center of the tube. As chromium chloride anhydrous is formed it sublimes and is carried in the nitrogen stream to the cooler far end of the tube, where it condenses. A byproduct of the reaction is poisonous phosgene gas, cobalt(II) chloride. [1]

Precursor of a Coordination Complex
The reaction of chromium chloride anhydrous and phenylmagnesium bromide in ether or in tetrahydrofuran is necessarily a heterogeneous one owing to the extreme insolubility of this metallic halide in organic solvents. Inorganic salts of chromium are in fact generally insoluble in all nonhydroxylic solvents; and this property presents difficulties in promoting their reactions in organic solvents with organic reagents. The study have now found that the trichloride may be made soluble by complexing it with tetrahydrofuran and that its reactions with Grignard reagents proceed rapidly, quantitatively, and homogeneously in this form. [2]
Modification of Coordination Complexes
CrCl3 (thf)3 has been widely used as a starting material for the synthesis of organometallic and coordination compounds of Cr. Its preparation method was reported 60 years ago; Chromium chloride anhydrous, which is insoluble in organic solvents as well as in water, was extracted with THF by the aid of Zn dust using Soxhlet apparatus. However, chromium chloride anhydrous is prepared by hazardous and not-facile process (treatment of Cr2O3·xH2O with CCl4 at 630 ◦C leads to the generation of extremely toxic phosgene gas), and thus, is expensive. Another method was reported in which chromium(III) chloride hexahydrate was reacted with excess thionyl chloride to generate chromium chloride anhydrous. In this process, a different form of hygroscopic CrCl3 was generated, from which CrCl3 (thf)3 could be immediately obtained after adding THF. The most attractive and convenient method is reacting chromium(III) chloride hexahydrate with excess Me3SiCl in THF, resulting in the deposition of CrCl3 (thf)3 along with the generation of byproducts, HCl and Me3SiOSiMe3 (CrCl3·6H2O + 12 Me3SiCl →CrCl3(thf)3 + 12 HCl + 6 Me3SiOSiMe3). Excess Me3SiCl (~60 equiv./Cr) needs to be added to obtain a completely anhydrous form of CrCl3(thf)3. Otherwise (e.g., when 25 equiv. of Me3SiCl/Cr was added according to the literature), hydrated CrCl3 (thf)2(H2O) is obtained, which was erroneously sold as CrCl3(thf)3 in the past. [3]
Safety and Risk
Chromium (III) can be potentially toxic to cells and induce a number of morphological and biochemical changes. This metal, often encountered in the form of chromium chloride anhydrous (CrCl₃), is widely used in many industries and can cause environmental pollution. It is also present in dietary supplements, vitamin and mineral products, and energy drinks. Moreover, chromium chloride anhydrous is used in dentistry and orthopedics as a component of implants, raising concerns about its biological safety. The present study analyzed the genotoxic effects of chromium chloride anhydrous on two cell lines: mouse embryo fibroblast cell line BALB/3T3 and human hepatocellular carcinoma cell line G2 (HepG2). The BALB/3T3 and HepG2 cell lines were exposed to chromium (III) chloride at concentrations ranging from 100 to 1400 µM, and the genotoxicity assays employed were the comet and micronucleus assays. On the basis of the initial results, selected concentrations of chromium (III) chloride were further tested in mixtures with cobalt chloride to examine combined effects. The results demonstratethatchromium chloride anhydrous induces genotoxic effects. [4]
References:
[1] https://alpha.chem.umb.edu/chemistry/ch371/documents/CrCl3.pdf
[2] W Herwig and H Zeiss. The Journal of Organic Chemistry 1958 23 (9), 1404-1404 DOI: 10.1021/jo01103a627.
[3] Lee, D. G., Baek, J. W., Lee, J. H., Lee, H. J., Seo, Y. H., Lee, J., ... & Lee, B. Y. (2021). Replacement of the common chromium source CrCl3 (thf) 3 with well-defined [CrCl2 (μ-Cl)(thf) 2] 2. Molecules, 26(4), 1167.
[4] Czarnek, K., Tatarczak-Michalewska, M., Blicharska, E., Siwicki, A. K., & Maciejewski, R. (2025). Genotoxic Effects of Chromium(III) and Cobalt(II) and Their Mixtures on the Selected Cell Lines. International Journal of Molecular Sciences, 26(11), 5056. https://doi.org/10.3390/ijms26115056.
You may like
See also
Lastest Price from Chromium(III) chloride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 10025-73-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00/kg2025-03-03
- CAS:
- 10025-73-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS