Application research of guaiazulene
Introduction
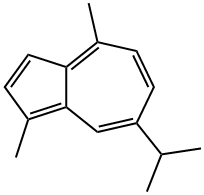
Guaiazulene (GA;Figure.1), a naturally occurring lipid-soluble azulene derivative used in cosmetics, baby skincare, and makeup products, is derived from plants like Matricaria chamomilla L., Callis intratropica blue, and guaiac wood oil of Guaiacum officinale oil. These plants are primarily found in South America's northern coast or the Caribbean region. In 1949, Plattner was the first person who elucidates the chemical structure of guaiazulene. Chemically guaiazulene (1,4-dimethyl-7-propan-2- ylazulene) is a sesquiterpene bicyclic compound substance found as a primary pigment in various soft corals. It has a molecular formula of C15H18 with a molecular mass of 198.3 g/mol and a melting point is 31.5℃. Azulon, vetivazulen, azunol, guajazulene, kessazulen, and azulol are some other names for guaiazulene that are frequently used. It is one of the azulene compounds investigated for pharmacological effects in various illnesses in the past 40 years. It has antiseptic, antiinflammatory, antimicrobial, antioxidant, epithelializing, anti mutagenic, immunomodulatory, fungicidal, expectorant, diuretic, diaphoretic, demulcent, and bitter stimulant properties. Addition ally, it is used to cure gastritis and canker sores. It is a potent antioxidant that can scavenge hydroxyl radicals and inhibit lipid per oxidation in the rat hepatic microsomal membrane. [1]

Effect of guaiazulene on radical-mediated processes
The effect of guaiazulene, a lipophilic azulene derivative widely found in nature, on radical-mediated processes is examined. The ability of guaizulene to inhibit rat hepatic microsomal membrane lipid peroxidation and to scavenge hydroxyl radicals, as well as to interact with 1,1-diphenyl-2-picrylhydrazyl radical (DPPH), was estimated. It was found that guaiazulene can inhibit lipid peroxidation very significantly, having an IC50 value of 9.8 microM. It can also scavenge hydroxyl radicals and interact with DPPH. The protection afforded by guaiazulene to rats with paracetamol-induced liver injury was also investigated. Paracetamol hepatotoxicity is caused by the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which causes oxidative stress and glutathione (GSH) depletion. Hepatic cytosolic protein, GSH, glutathione transferase and glutathione reductase levels are determined as indices of hepatic injury with or without the administration of guaiazulene. It was found that all parameters affected by paracetamol are restored to normal by guaiazulene treatment, while the administration of guaiazulene alone has no effect on the performed tests compared with the control values. It was concluded that the significant protection against paracetamol-induced GSH depletion and hepatic damage afforded by guaiazulene is probably connected with its antioxidant activity. A molecular mechanism of action of guaiazulene is suggested.[2]
Guaiazulene Derivatives study
Guaiazulene and related derivatives were famous for diverse biological activities. In an effort to discover new highly efficient candidate drugs derived from guaiazulene, four series of guaiazulene derivatives were designed, synthesized, and evaluated for antiproliferation, antiviral, anti-inflammatory and peroxisome proliferators-activated receptor γ (PPARγ) signalling pathway agonist activities. Among them, two guaiazulene condensation derivatives showed selective cytotoxic activities towards K562 cell with IC50 values 5.21 μM and 5.14 μM, respectively, accompanied by slight effects on normal cell viability. For the first time, one guaiazulene derivative from series I exhibited potent antiviral activity towards influenza A virus with IC50 of 17.5 μM. A guaiazulene-based chalcone showed higher anti-inflammatory activity than positive drug indomethacin with an inhibitory rate of 34.29 % in zebrafish model in vivo. One guaiazulene-based flavonoid could strongly agitate PPARγ pathway at 20 μM, indicating the potential of guaiazulene derivatives to reduce obesity development and ameliorate hepatic steatosis. Preliminary in silico ADME studies predicted the excellent drug-likeness properties of bioactive guaiazulene derivatives.[3]
Toxicity of Guaiazulene and its derivatives
Bentazone, a non-toxic derivative of guaiazulene, has been proven in acute intoxication studies on sheep. In a study for 84 days, bentazone with sunflower oil (1:5) at doses of 175 mg/kg (1/10th of its LD50) and 97.5mg/kg (1/12th of its LD50) negatively impacted haemoglobin, leukocyte, and erythrocyte count. In vivo study on mice, cadalene derivative has shown antioxidant and chemopreventive effects at 100mg/kg, orally without any other harmful toxicant effects. However, it has a major problem due to its insolubility in water. Guaiol has shown good insecticidal activity at the concentration of 70 mg/L and good contact activity against insect larvae of Mythimna separate and Plutella xylostella with LD50 of 0.07 and 8.9 mg/larva. Guaiene has potential contact toxicities against cigarettes, red flour beetle, andbooklouse with LD50 17.9 μg/adult.[1]
Side effects of Guaiazulene and its derivatives
Guaiazulene has no systemic and local side effects after locally applied on the skin in diaper dermatitis. In vitro study of guaiazulene on phototoxic features and cytotoxicity has shown no harmful adverse effects. However, minor side effects, such as allergic contact cheilitis,were seen after using guaiazulene-containing toothpaste. A study on bentazone has confirmed that it is non-toxic in honeybees and beetles. However, it causes allergic side effects, such as skin, eyes, and respiratory tract irritation. It also shows severe side effects such as acute renal and respiratory failure on large doses. Guaiol has no side effects due to its non-irritating, non-toxic, and non-sensitizing properties. Guaiene has no specific side effect but increases body weight on higher doses.[1]
Guaiazulene in Non-small Cell Lung Cancer
Non-small cell lung cancer (NSCLC) is one of the most frequent cancers worldwide, yet effective treatment remains a clinical challenge. Guaiazulene (GYZ), a cosmetic color additive, has previously been characterized as a potential antitumor agent due to observed anticancer effects. However, the efficacy of guaiazulene in the treatment of NSCLC and the involved molecular mechanisms remain largely unknown. Here, we indicated a role for guaiazulene in the suppression of NSCLC both in vitro and in vivo via triggering reactive oxygen species (ROS)-induced apoptosis. Concomitantly, guaiazulene induced complete autophagic flux in NSCLC cells via inhibiting the Akt/mTOR signaling pathway, which displayed cytoprotective effect against GYZ-induced growth suppression. Accompanied with autophagy inhibition obviously enhanced the effects of guaiazulene. Notably, guaiazulene acts synergistically with paclitaxel in the suppression of NSCLC in vitro. Together, our results for the first time reported that guaiazulene suppressed the proliferation of NSCLC and suggested a potential strategy for inhibiting NSCLC growth by combinational use of guaiazulene and autophagy inhibitors.[4]
References
[1] Akram W, Tagde P, Ahmed S, et al. Guaiazulene and related compounds: A review of current perspective on biomedical applications. Life Sci. 2023;316:121389. doi:10.1016/j.lfs.2023.121389
[2] Kourounakis AP, Rekka EA, Kourounakis PN. Antioxidant activity of guaiazulene and protection against paracetamol hepatotoxicity in rats. J Pharm Pharmacol. 1997;49(9):938-942. doi:10.1111/j.2042-7158.1997.tb06140.x
[3] Ma Z, Han X, Ren J, Liu K, Zhang W, Li G. Design, Synthesis, and Biological Activity of Guaiazulene Derivatives. Chem Biodivers. 2023;20(2):e202201174. doi:10.1002/cbdv.202201174
[4] Ye Q, Zhou L, Jin P, et al. Guaiazulene Triggers ROS-Induced Apoptosis and Protective Autophagy in Non-small Cell Lung Cancer. Front Pharmacol. 2021;12:621181. Published 2021 Apr 15. doi:10.3389/fphar.2021.621181
You may like
See also
Lastest Price from Guaiazulene manufacturers

US $10.00/ASSAYS2025-08-19
- CAS:
- 489-84-9
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00-0.00/KG2025-06-10
- CAS:
- 489-84-9
- Min. Order:
- 0.0001KG
- Purity:
- 99.99%
- Supply Ability:
- 2000000T