Application research of 2-(dicyclohexylphosphino)biphenyl
Introduction
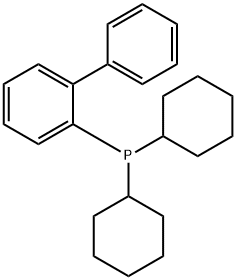
2-(Dicyclohexylphosphino)biphenyl (Figure 1) is an important organic compound, which is widely used in chemical synthesis and materials science. It belongs to biphenyl compounds, and its molecular structure contains two benzene rings and a dicyclohexylphosphine group. Dicyclohexylphosphine group refers to a phosphorus group containing two cyclohexane structures, which makes 2-(dicyclohexylphosphino)biphenyl show unique properties in chemical reactions. This structure plays an important role in the design and synthesis of catalysts.

Influence of biaryl phosphine structure on C-N and C-C bond formation
In order to understand how electronic and other structural characteristics of biphenyl phosphine ligands affect Pd-catalyzed C-N and C-C bond-forming reactions, a new ligand, 2-(dicyclohexylphosphino)-4'-(N,N-dimethylamino)-1,1'-biphenyl, was synthesized. This compound is isomeric with the commercially available 2-(dicyclohexylphosphino)-2'-(N,N-dimethylamino)-1,1'-biphenyl that has been useful in C-N bond-forming reactions of nucleosides. The new p-dimethylamino biphenyl ligand bears electronic similarities to the o-dimethylamino isomer, but it also possesses structural similarities to 2-(dicyclohexylphosphino)biphenyl, such as the unsubstituted ortho positions in the non-phosphine ring. Whereas 2-(dicyclohexylphosphino)biphenyl can support catalysts for C-C bond formation, it was not effective in promoting aryl amination of a nucleoside substrate. However, the new ligand proved to be effective in promoting both aryl amination and C-C bond-forming reactions of nucleoside substrates, with some reactions even occurring at room temperature. Thus, the composite structural elements of this new ligand are thought to be criteria for reactivity of the catalytic system derived from it. Researchers have probed the structures of the isomeric N,N-dimethylamino biphenyl ligands by X-ray crystallographic analysis. Interactions of the two ligands with Pd(OAc)(2) have been investigated by (31)P NMR, and they show substantial stoichiometry-dependent differences. These results have been compared to the interactions of Pd(OAc)(2) with 2-(dicyclohexylphosphino)biphenyl as well as 2-(di-tert-butylphosphino)biphenyl, and they reveal marked differences as well. In this process, three cyclopalladated biaryl derivatives have been isolated and characterized by X-ray analysis.[1]
Cross coupling reactions of hypervalent siloxane derivatives
The formation of carbon-carbon bonds by palladium-catalyzed cross coupling reactions is one of the fundamental reactions in the arsenal of synthetic organic chemistry.The traditional methodologies most often used to accomplish these types of transformations are the Stille and Suzukicoupling reactions.
The scope of the palladium-catalyzed cross coupling reaction of aryl halides with phenyltrimethoxysilane has been expanded to include aryl bromides, heteroaryl bromides, and aryl chlorides. A more general Pd(0)-catalyst/ligand system has been developed to activate bromides: palladium(II) acetate (Pd(OAc)2) is activated with triphenylphosphine (PPh3) or tri-o-tolylphosphine (P(o-tol)3) (1:2 molar ratio of Pd:phosphine). Coupling of aryl chloride derivatives required addition of 2-(dicyclohexylphosphino)biphenyl (Buchwald's ligand) to Pd2dba3 (tris-(dibenzylideneacetone)dipalladium(0)) (1:1.5 molar ratio of Pd:phosphine).[2]
Palladium-catalyzed indole, pyrrole, and furan arylation by aryl chlorides
The palladium-catalyzed direct arylation of indoles, pyrroles, and furans by aryl chlorides has been demonstrated. The method employs a palladium acetate catalyst, 2-(dicyclohexylphosphino)-biphenyl ligand, and an inorganic base. Electron-rich and electron-poor aryl chlorides as well as chloropyridine coupling partners can be used, and arylated heterocycles are obtained in moderate to good yields. Optimization of base, ligand, and solvent is required for achieving best results.Unfortunately, it appears that at this point arylationby unactivated aryl chlorides requires extensive optimizationof reaction conditions for every substrate class to determineoptimal ligand, base, and solvent.N-Arylation of indoles and pyrroles is preferred to C-arylation if unactivated arylchloride coupling partners are employed. Further investigations are required to solve these problems.[3]
Obtain Arylpyridines and Arylquinolines
Suzuki-Miyaura coupling is a well-known synthetic tool for C-C bond formation. This reaction has often been used to obtain a wide variety of biaryl compounds,which are used in pharmaceuticals, herbicides, and light-emitting materials. In such cases, homogeneous palladium complexes are generally used as a catalyst.However, the reaction with a heterogeneous catalyst such as Pd/C has also recently been investigated. Compared to air-sensitive and expensive homogeneous palladium catalysts, palladium charcoal can be safely handled and removed from the reaction mixture by simple filtration.The recovered palladium charcoal can be purified and reused as palladium metal. These features are great advantages in an industrial process.
A phosphine ligand, such as PPh3 or 2-(dicyclohexylphosphino)biphenyl, is essential for the Pd/Ccatalyzed Suzuki-Miyaura coupling of halopyridines and haloquinolines, although it has been reported that the reaction of phenyl chlorides can be catalyzed by nonprereduced Pd/C without any additives. In the reactions of bromopyridines, bromoquinolines, 2-chloropyridines, and 2-chloroquinolines, PPh3 was effective enough to provide coupling products in good yields. However, in the reactions of 3-chloropyridine, 4-chloropyridine, and 6-chloroquinoline, sterically hindered 2-(dicyclohexylphosphino)biphenyl was indispensable as a ligand.[4]
References
[1] Pratap R, Parrish D, Gunda P, Venkataraman D, Lakshman MK. Influence of biaryl phosphine structure on C-N and C-C bond formation. J Am Chem Soc. 2009;131(34):12240-12249. doi:10.1021/ja902679b
[2] Mowery ME, DeShong P. Improvements in cross coupling reactions of hypervalent siloxane derivatives. Org Lett. 1999;1(13):2137-2140. doi:10.1021/ol991186d
[3] Nadres ET, Lazareva A, Daugulis O. Palladium-catalyzed indole, pyrrole, and furan arylation by aryl chlorides. J Org Chem. 2011;76(2):471-483. doi:10.1021/jo1018969
[4] Tagata T, Nishida M. Palladium charcoal-catalyzed Suzuki-Miyaura coupling to obtain arylpyridines and arylquinolines. J Org Chem. 2003;68(24):9412-9415. doi:10.1021/jo034970r
You may like
See also
Lastest Price from 2-(Dicyclohexylphosphino)biphenyl manufacturers

US $10.00/KG2025-04-21
- CAS:
- 247940-06-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00/Kg2025-04-18
- CAS:
- 247940-06-3
- Min. Order:
- 1Kg
- Purity:
- 98%; sales036@researchvip.com;18903931628;
- Supply Ability:
- 100kg