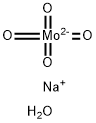Sodium Molybdate Dihydrate: Applications, Oyster Shell Growth & Toxicity Profile
Sodium molybdate is an excellent source of molybdenum, a pure white, free-flowing, crystalline salt that’s commonly available as the dihydrate (Na2MoO4·2H2O). Traditionally, sodium molybdate was produced by the process of hydration. Another convenient way to prepare sodium molybdate is by using sodium hydroxide to dissolve MoO3 at the levels of 50 – 70 ° C and crystallise the residue. As a chemical compound, Sodium molybdate dihydrate is used in a wide range of applications in different industries, ranging from agriculture to industrial sectors. Below, we discuss some of the most popular applications of sodium molybdate. In the agricultural sector, Sodium molybdate dihydrate is used to supply the element of molybdenum to both plants and livestock. It serves as a fertiliser for crops and vegetables, for example cauliflower. This supplement is recommended for treating whiptail in molybdenum-deficient soils for certain crops, such as broccoli. Sodium molybdate serves as a metal corrosion inhibitor in industrial applications due it non-oxidizing anodic properties. Furthermore, adding Sodium molybdate dihydrate can enhance the corrosion protection of carboxylate salt fluids as well as reducing the need for nitrites in fluids impeded with nitrite-amine. In the food sector, Sodium molybdate is used as a nutritional supplement. Most individuals don’t require an additional supply of molybdenum as it’s already present in various foods like potatoes, beef liver, cheese, whole-grain bread, yoghurt, corn, tuna, legumes, and more.

Sodium molybdate dihydrate increases shell growth in the Pacific oyster Magallana gigas
To test of the role of sulfate reducers in shell formation, we reared larvae of Pacific oyster (Magallana gigas, formerly Crassostrea gigas) through settlement while exposing them to pulses of Sodium molybdate dihydrate, a compound that has been used in previous experiments to inhibit sulfate-reducing bacteria in marine systems. A previous study showed that the abundance of sulfate-reducing bacteria in juvenile oysters is not correlated with enhanced chalk expression, but is correlated with denser shells. Larvae were raised under two experimental treatments: control seawater (pH≈8.00) and seawater with Sodium molybdate dihydrate added. As a functional analog of the sulfate ion, molybdate can be transported into bacterial cells during cellular respiration, which deprives the microbe of sulfur, thus acting as a sulfate-reduction inhibitor. Each treatment was maintained in three 10-gallon food-grade buckets, which were pretreated for 3 days, with the water changed each day. Filtered seawater from the Bodega Marine Lab intake system was pretreated with air bubbled through an airstone. Thus, oysters exposed to sodium molybdate were larger in shell area than the control, and buckets played a large role in explaining the variation between individuals within treatments. As Sodium molybdate dihydrate was added to each treatment bucket separately, we suspect this was the primary cause of variation between treatment buckets, which contributed to observed variation in oyster growth.[1]
The primary goal of this experiment was to characterize the effects of sodium molybdate on the Pacific oyster M.gigas. Our a priori hypothesis was that Sodium molybdate dihydrate would competitively replace sulfate ions, inhibiting activity by sulfate-reducing bacteria and potentially leading to decreased oyster growth due to the influence of these bacteria on calcification. However, oysters grew larger when exposed to sodium molybdate compared to the control group, contradicting our initial hypothesis. In a previous paper using sodium molybdate on adult oysters, found that Sodium molybdate dihydrate treated animals had shells that were significantly more dense than control animals (with mean densities of 2.31 gcm-3 and 1.92 gcm-3, respectively). Many of the differences observed between sodium molybdate and control oysters at 38 days post-fertilization diminished by 51 days. Bacterial community composition (beta diversity) varied between treatments at 38 days post-fertilization but no longer differed by 51 days. Finally, transcriptome analyses suggest that gene expression profiles of oysters exposed to sodium molybdate are distinct from the control at 38 days, but overlap by 51 days. Most enriched GO terms were found when comparing Sodium molybdate dihydrate treated time points, and were primarily related to metabolism and responses to other organisms.
Accidental ingestion of sodium molybdate dihydrate at the workplace
We recently had to deal with a case of accidental ingestion of molybdenum. A 36-year-old man, worker at a company that deals with boiler maintenance, accidentally ingested during worktime a complete sip of a pure industrial product, used for metal protection, that contained Sodium molybdate dihydrate (Mo(VI)). The sip was ingested secondary to incautious aspiration of the product from a boiler tube in which it had been put in excess. The product was a liquid substance, with a pH of 7, used to avoid corrosive phenomena caused by water over metals (particularly copper, alluminium and steel) and to prevent encrustation and gas formation in hydraulic pipes. The safety data sheet of the product was analyzed and revealed this composition: sodium molybdate at a maximum concentration of 10% and triethanolamine at a maximum concentration of 4%. We estimated the volume of the sip around 50 ml, with an estimation of a total of 5 gr of Sodium molybdate dihydrate that, for the patient bodyweight of 80 kg, would mean 62,5 mg/kg of Mo.[2]
At emergency department (ED) admission he presented asymptomatic, and the baseline biochemical investigations were normal. Due to the lack of clinical data about acute molybdenum exposure, he was hospitalized to monitor the possible development of clinical manifestations and/or biochemical alterations. Blood and urine samples were collected 2 hours after ingestion to perform molybdenum determinations that resulted 50 mcg/L (reference range: 0.43 – 1.8 mcg/L) and 630 mcg/L (refence range: up to 116 mcg/L), respectively. Beside Sodium molybdate dihydrate, the ingested substance contained triethanolamine that is considered a mild irritant for humans and has been studied for the possible carcinogenic effects associated with chronic exposure, without finding any positive association (IARC group 3 carcinogen). Moreover, its irritative effect appears with a concentration of 5% or more, so that the product ingested by the patient, with a maximum concentration of 4%, would lack the possible irritative effects specifically associated with triethanolamine. Finally, studies conducted on rats on triethanolamine toxicity allowed the calculation of a NOAEL of 9000-10000 mg/kg/day, which is higher than the estimated ingested dose of our patient (25mg/kg).
References
[1]Banker RMW, Lipovac J, Stachowicz JJ, Gold DA. Sodium molybdate does not inhibit sulfate-reducing bacteria but increases shell growth in the Pacific oyster Magallana gigas. PLoS One. 2022 Feb 9;17(2):e0262939. doi: 10.1371/journal.pone.0262939. PMID: 35139090; PMCID: PMC8827440.
[2]Bernasconi L, Brolli B, Negro A, Zoino JL, Schicchi A, Petrolini VM, Lonati D, Ronchi A, Locatelli CA. Accidental ingestion of sodium molybdate at the workplace followed by short-term biomonitoring. Med Lav. 2022 Apr 26;113(2):e2022015. doi: 10.23749/mdl.v113i2.12877. PMID: 35481580; PMCID: PMC9073763.
You may like
Lastest Price from Sodium molybdate dihydrate manufacturers

US $0.00-0.00/KG2025-04-22
- CAS:
- 10102-40-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1000kg/month

US $0.00-0.00/KG2025-04-22
- CAS:
- 10102-40-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20TONS