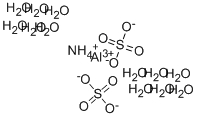
Chemical Properties
Colorless transparent cube or white powder. No smell, astringent taste. Slightly soluble in water (15g/100ml at 20℃), easily soluble in hot water. The water solution is acidic. Soluble in dilute acid and glycerol, insoluble in alcohol. Loses 10 molecules of water when heated to 120℃, becomes anhydrous alum when heated to 200℃, and decomposes at 280℃.
Uses
Ammonium aluminum sulfate dodecahydrate may be used in the preparation of alum (Al(OH)
3) gel and monodispersed yttrium aluminum garnet (YAG) powder.
Definition
ChEBI: Ammonium alum is an inorganic sulfate salt.
Preparation
Aluminium ammonium sulfate dodecahydrate is produced through the reaction of sulphuric acid with aluminium hydroxide. Ammonium sulphate is added to the mixture and allowed to react. The mixture is then cooled and crystallized, followed by centrifugal separation. The resulting product is dried to obtain the final aluminium ammonium sulfate.
General Description
Ammonium aluminum sulfate dodecahydrate, commonly called as ammonium alum, is widely used in the water purification, cosmetic formulation, fertilizers and food preservation. Its thermal decomposition has been studied. It has high latent heat of 251kJkg
-1 with a potential use in the heating system. Its utility as a flame-retardant for cotton fabric has been studied.
Purification Methods
Crystallise it from hot H2O and cool in ice. When the melt is heated, it loses NH3 and H2SO4, and gives pure alumina at red heat. Solubility (%) in H2O is 3.9 (0o), 15.0 (20o) and 135 (100o).