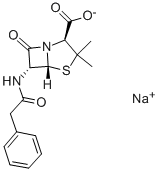
Penicillin G sodium salt
- Product NamePenicillin G sodium salt
- CAS69-57-8
- CBNumberCB9211940
- MFC16H17N2NaO4S
- MW356.37
- EINECS200-710-2
- MDL NumberMFCD00069666
- MOL File69-57-8.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 209-212°C |
| alpha | D24.8 +301° (c = 2.0 in water) |
| Density | 1.41 |
| refractive index | 300 ° (C=2, H2O) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | H2O: 100 mg/mL Solutions should be filter sterilized and stored at 2-8°C for 1 week or at -20°C for extended periods. Solutions are stable at 37°C for 3 days. |
| form | powder |
| color | colorless or white |
| PH | 5.5-7.5 (3% in H2O) |
| Water Solubility | 5-10 g/100 mL at 25 ºC |
| Merck | 14,7094 |
| BRN | 3834217 |
| Stability | Stability Stable, but incompatible with a wide variety of materials, including acids, oxidizing agents, heavy metals, alcohols, glycerol, thiomersal, many surface-active agents, alkalies, peroxides, reducing agents, lanolin, glycol, sugars, amines, iodine, iodides, thiols. Hygroscopic. |
| CAS DataBase Reference | 69-57-8(CAS DataBase Reference) |
| FDA 21 CFR | 556.510 |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | penicillin |
| FDA UNII | YS5LY7JF4N |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H317 | |||||||||
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P362+P364 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 42/43 | |||||||||
| Safety Statements | 22-36/37-45 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XH9800000 | |||||||||
| F | 10-23 | |||||||||
| HS Code | 29411000 | |||||||||
| Toxicity | LD50 oral in rat: 6916mg/kg | |||||||||
| NFPA 704: |
|